Although the terms sound practically the same, absorption and adsorption are vastly different. The major distinction between absorption and adsorption is that absorption is the process by which a liquid or solid dissolves a fluid. Adsorption occurs when atoms, ions, or molecules from a substance cling to the adsorbent’s surface. Atoms travel through or enter a big substance during the absorption process. The molecules are held loosely on the surface of the adsorbent during adsorption and can be easily removed.
Absorption
Absorption occurs when one substance penetrates the volume or bulk of another material. Rather than any forces acting on molecules, the solid absorbs the liquid or gas. The absorbate is the stuff that is absorbed, while the absorbent is the substance that absorbs. Absorption can take place in a chemical or physical manner.
Adsorption
Adsorption is the phenomenon of molecules of a material adhering to the surface of a liquid or solid. The adsorbate is the material that gets adsorbed on a surface, while the adsorbent is the substance on which it is adsorbed. The process takes place on the surface of the interface. There are two types of adsorption: physisorption and chemisorption.
Difference between adsorption and absorption
The Difference Between Absorption And Adsorption
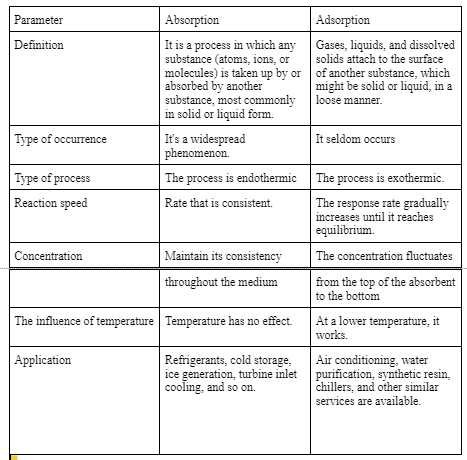
Different Types of absorption
1.Chemical absorption, also known as reactive absorption, is a chemical reaction that occurs between the absorbed and absorbing substances.
2.Physical absorption- The electronic structure of the atom or molecule is minimally altered during this process. When oxygen from the air dissolves in water, it is a non-reactive process.
Different types of adsorption
1.Chemical adsorption-;When gas molecules are linked to the surface by a chemical bond, this is known as chemical adsorption or chemisorption.
2.Physical Adsorption-:When gas molecules are bound by the total of attraction and repulsive interactions between molecules, as well as electrostatic forces, physical adsorption, also known as physisorption, occurs.
Application of Adsorption
Adsorption has a wide range of applications.
1.When it comes to vacuum preservation,
2.When it comes to softening hard water,
3 In the paint business, in dyeing, and in the separation of inert gases in the froth flotation process.
Application of Absorption
The following are two examples of adsorption application
(I)To eliminate dangerous gases such as carbon monoxide and methane, activated charcoal is utilised in gas masks. Animal charcoal is used in the sugar industry to remove colourants from sugarcane juice.
(ii) To reduce hardness from water, ion exchange resin is utilisedethane, activated charcoal is utilised in gas masks. Animal charcoal is used in the sugar industry to remove colourants from sugarcane juice.
(ii) To reduce hardness from water, ion exchange resin is utilised
Conclusion
We conclude that The processes of absorption and adsorption are not the same. Both absorption and adsorption are physico-chemical processes in which a tiny amount of one substance enters or binds to a larger amount of another substance.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



