Phenols are the natural compounds containing a benzene ring bonded with a hydroxyl group. They are even called carbolic acids. Hence, a phenol molecule contains two parts: one aryl group part and the other is hydroxyl group part.
Naming of Phenols
Phenol includes two elements: one aryl group and one hydroxyl group. So primarily based on the number of aryl groups connected, Phenols may be further sub-labeled as mono-, di-, tri-, or polyhydric Phenols. The IUPAC name for Phenol is Mono hydroxy benzene or can be called C6H5OH.
Rules for Nomenclature
Earlier than progressing similarly to the nomenclature of Phenols, let us revise some essential regulations of IUPAC nomenclature that are well known.
According to the hints of IUPAC, the following rules are vital to comply with when practicing nomenclature-:
- The longest chain rule: As per this rule, parent hydrocarbons ought to be identified and classified for that reason.
- The lowest set of locants: This rule implies the numbering of the carbon atoms that belong to the figure hydrocarbons.
- More than one instance of the same substituent: This rule refers to the usage of prefixes which include di-, tri-, mono-, or poly.
- The naming of different substituents: Alphabetical order should be observed for special substituents in a single compound.
Nomenclature for Phenols
IUPAC has a fixed set of pointers in a location that needs to usually be observed even as naming Phenols. This is finished to ensure uniformity for all practical purposes.
- The IUPAC name of Phenol is benzenol. Whenever a molecule of the hydroxyl group, denoted via OH, is attached to a hoop of benzene, the resulting shape is a Phenol.
- In case there are greater than one of these hydroxyl groups, then the benzene ring is marked with di-, tri-, or poly, whoever prefix works for it and may correctly denote the variety of hydroxyl organizations that can be attached to these rings. Let’s say, there are three hydroxyl groups, then the nomenclature could be benzene, 1,2,3-diol.
- There are some functional groups that are attached to the Phenol molecule. These functional groups are carbonyls, carboxylic acids, amines, alcohols, and so forth. Those can be used to alternate the characteristics of the molecule. Functional corporations can therefore be connected to Phenol molecules. The methyl group is a superb example of this.
- The nomenclature for the Phenol structure above is 4-methyl Phenol. Furthermore, relying on which this functional group is attached, the labeling of the compound will fluctuate.
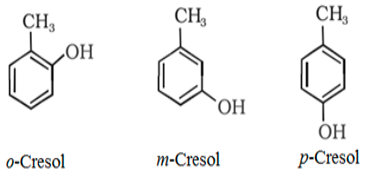
- For instance, if the functional group, methyl, turns into ortho (connected to the adjacent carbon) then the name of the compound will be exceptional from when it is attached to para (attached to the 0.33 carbon of the hydroxyl carbon).
- Every other common position wherein the functional group can be attached is meta (attached on the second carbon from the hydroxyl group). You should usually recollect the position of OH as it will give an excessive priority.
Benzene Ring
Benzene is an organic chemical compound. It is classified as a benzenoid aromatic compound. All the compounds which have a benzene ring and are stable are called the benzenoid aromatic compound. On the other hand, compounds that do not have benzene ring but are stable are called non-benzenoid aromatic compounds.
Conclusion
Phenols are organic compounds that contain a benzene ring bonded to a hydroxyl group. They are also called carbolic acids. Phenols are weak acids and are not completely soluble in water. They also have many applications in households, medical and industrial fields. Above we have discussed the nomenclature of phenols and what are the rules of the IUPAC nomenclature. We have talked about 4 methyl- Phenol and benzene rings.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




