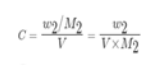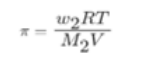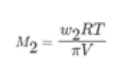Numerous substances and elements in our environment offer a rare opportunity to learn about their properties. Our understanding of these substances will be improved if we investigate their various properties. Molar mass is the most important factor in understanding the properties of these substances. Whatever the state of a substance is, it tends to behave in a certain way, largely determined by its properties. This means that the colloidal properties of all liquid substances can be examined. The vapour pressure of the solution can then be measured using this technique. So, let’s look at how matter interacts with one another and how molar mass is calculated.
Method to determine the molar masses
The simplest method for determining a compound’s molecular weight has been to use the solution’s colloidal properties. Molecular mass measurements of proteins, macromolecules, and polymers can all be determined using this method, which relies on the colligative properties of solutions.
The substance is dissolved in a solvent with a known boiling point, freezing point, or vapour pressure. Under the given conditions, the property to be measured is chosen so that its measurement is straightforward and does not vary significantly.
Colligative Properties of Liquids
The properties of a liquid that are dependent on the number of solute particles present, rather than the solution’s concentration, are known as coagulative properties. Liquids are used to investigate these properties. These properties indicate that when a nonvolatile solution is mixed with a volatile solvent, the relative vapour pressure of the solution is reduced. As a result of this decrease in vapour pressure, the properties of all liquid solutions can be quantified and studied in greater detail. Specifically, the solute particles in a given solution impact the colloidal properties of a solution. It is derived from Latin ‘Coligare,’ which means “to bind together.” Osmosis and osmotic pressures have four colligative properties: lower vapour pressure, higher boiling point, and lower freezing point.
Relative Lowering of Vapour Pressure
Vapour pressure decreases with the addition of a nonvolatile solute. The decrease in vapour pressure is the cause of this phenomenon. A French chemist discovered a connection between the solution’s pressure, the pure solvent’s vapour pressure, and the solute’s mole fraction. He also observed that the lower vapour pressure is primarily due to the higher concentration of solute particles. Decrement in Vapour Pressure = Vapour Pressure of Pure Solvent – Vapour Pressure of Solvent, according to the scientific laws of the time. We can determine a solute’s ultimate molar mass using this equation.
Elevation of Boiling Point
When a nonvolatile solute is added to a solvent, the vapour pressure of the solute tends to fall. In this case, it is important to note that the boiling point of the solution is always higher than that of the pure solvent. Because the vapour pressure is directly proportional to the solution’s temperature, this occurs.
To boil, you’ll need to raise the temperature of the mixture. When this happens, it’s known as a rise in the boiling point. Together, solute particles and vapour pressure contribute to this phenomenon. The boiling point rises when a nonvolatile solute is added to a pure solvent. Because the solute concentration in the solution is directly related to the boiling point in increases, mathematically, it is given as:
∆Tb = Kbm
Where
∆Tb = elevation in boiling point
Kbm= Boiling Point Elevation Constant
Depression of Freezing Point
Reduced freezing is another effect of lowering the vapour pressure of a solution. Whenever the vapour pressure of a given substance is equal in its liquid and vapour states, the freezing point of a solution can be determined. As with the boiling point, the freezing point of a given diluted solution is also determined by the molality of the solute. We can also determine the molar masses of various substances by lowering the freezing point of solutions, which is another colligative property. We know that depression at a freezing point is given as:
∆Tf = Kfm
where ∆Tf = depression at freezing point
Kfm = freezing Point depression constant
Osmotic Pressure
In the past, you may have noticed the shrivelling of raw mangoes when placed in salt water or the revival of wilted flowers when placed in freshwater. Osmosis is responsible for this phenomenon. Molecules in a solution are transported to and from the solution using the process of osmosis Equilibrium is reached when the flow is at its maximum.
Small solvent molecules, such as water, can pass through a membrane that appears to be continuous but contains small pores that allow larger solute molecules to pass through. Such membranes are referred to as semi-permeable membranes (SPM). OP stands for Osmotic Pressure, which is the pressure needed to stop solvent molecules from flowing through a semipermeable membrane as they move from a dilute solution to a concentrated one. Mathematically it is given as:
Π = CRT
Where,
π =osmotic pressure
C= Molar concentration of the solution
R= Universal gas constant
T= Temperature
Now molar concentration of a solution containing w2 grams of solute whose molar mass is m2
and the total volume of the solution is V litres, which can be written as:
So we can now write the osmotic pressure as:
Rearranging the above equation, we get,
Conclusion
Through this article, we understood how we could calculate the molecular weight using the colligative properties of solutions. The three methods discussed above provide us with the options used based on the type of substance, the solvent’s nature, and the degree of accuracy required during measurement.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out