Lewis structures, also known as Lewis dot formulas, Lewis dot structures, electron dot structures, or Lewis electron dot structures (LEDS), are diagrams that show the bonding between atoms in a molecule, as well as any lone pairs of electrons present. Lewis structures depict each atom and its place in the molecule’s structure by utilising the chemical symbol associated with that atom. Lines are drawn between atoms that are chemically or physically connected to one another (pairs of dots can be used instead of lines). lone pairs are formed by excess electrons in a molecule, and these lone pairs are depicted as pairs of dots next to the atoms.
Main group elements of the second period and beyond typically react by acquiring or losing electrons until they reach a valence shell electron configuration consisting of a whole octet of (8) electrons, however hydrogen (H) can only form bonds in which two electrons are shared.
The Construction of Structures And The Counting of Electrons
It is equal to the sum of the numbers of valence electrons present on each individual atom in a Lewis structure to reflect the total amount of electrons represented in a Lewis structure. Lewis structures do not contain any electrons that are not valence electrons.
The electrons must be placed into the structure after the total number of available electrons has been determined. The electrons must be placed into the structure in the following steps:
- Single bonds are used to join the atoms in the first place.
- If t is the total number of electrons and n is the number of single bonds, there are t-2n electrons that need to be positioned in their proper positions. Lone pairs should be used for these: one pair of dots for every two electrons that are now available. It is recommended that extra lone pairs be placed on the outer atoms (apart from hydrogen) to begin with, until each outer atom has eight electrons in bonding pairs and lone pairs; further lone pairs may then be placed on the core atom. Lone pairs should always be placed on the more electronegative atoms first, even if they aren’t necessary.
- Once all of the lone pairs have been inserted, it is possible that certain atoms (particularly the core atoms) will not have an octet of electrons. It is necessary in this scenario for the atoms to create a double bond, which is accomplished by moving a single pair of electrons between the two atoms. Because the bonding pair is shared between the two atoms, the atom that initially had the lone pair retains its octet, while the other atom gains two additional electrons in its valence shell as a result of the bonding pair.
Lewis structures for polyatomic ions can be drawn using the same method as for monatomic ions. When counting electrons, negative ions should have more electrons deposited in their Lewis structures than an uncharged molecule, and positive ions should have less electrons placed in their Lewis structures than a charged molecule. Typically, when writing the Lewis structure of an ion, the entire structure is enclosed in brackets, and the charge is represented as a superscript on the upper right, outside the brackets, of the structure.
When constructing Lewis structures, a simpler method has been proposed that eliminates the need for electronic tally. The atoms are drawn with their valence electrons clearly visible; bonds are formed by pairing up the valence electrons of the atoms involved in the bond-making process; then anions and cations are formed by adding or removing electrons from the appropriate atoms.
Resonance
It’s difficult to know which lone pairs should be relocated to form double or triple bonds for some molecules and ions, so two or more distinct resonance structures may be constructed for the same molecule or ion. It is customary to write all of them with two-way arrows in between in such instances (see Example below). When many atoms of the same sort surround the core atom, this might happen, and it’s especially common in polyatomic ions.
When this happens, the Lewis structure of the molecule is said to have a resonance structure, and the molecule is said to be a resonance hybrid. Each of the possible states is overlaid on the others, and the molecule is said to have a Lewis structure that is comparable to some combination of them.
To meet the octet rule for nitrogen, the nitrate ion (NO3), for example, must establish a double bond between nitrogen and one of the oxygens. It doesn’t matter which of the oxygens makes the double bond because the molecule is symmetrical. There are three conceivable resonance configurations in this scenario. You can indicate resonance in Lewis structures by drawing each conceivable resonance form and linking them with double-headed arrows, or by using dashed lines to represent partial bonds (although the latter is a good representation of the resonance hybrid which is not, formally speaking, a Lewis structure).
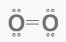
For The Oxygen Molecule, The Lewis Electron Dot Structure is Used
The bonding atoms, the number of bonds in the molecule, and the lone pairs left in the bonding atoms are all described by the Lewis electron dot structure.
Nitrogen’s Electronic Configuration
The arrangement of an element’s electrons in its atomic orbitals is known as its electronic configuration. Nitrogen has an atomic number of 7 and an electronic configuration of 1s22s22p3.
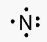
Conclusion
Lewis dot structures, also known as electron dot structures, depict the atomic bonding in a molecule. They also show the total number of lone pairs in each atom in the molecule.
Lewis structures (LEDS) are diagrams that illustrate the bonding between atoms in a molecule, as well as any lone pairs of electrons that may occur. Lewis structures are employed in physics and chemistry.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






