Organic compounds are called using the International Union of Pure and Applied Chemistry’s (IUPAC) six-step procedure, which includes a prefix, suffix, and root. Learn each step to gain a better understanding of the importance of names in giving information about organic compounds.
Carbon is an atom found in organic chemistry. Carbon is a one-of-a-kind atom because it can create incredibly stable molecules of varying sizes. Furthermore, carbon can form bonds with other atoms to form an almost limitless number of organic molecules. What makes this possible?
Organic compounds, by definition, contain a carbon atom. It’s important to remember that practically all organic molecules contain a hydrogen atom.
What role does the carbon atom have in the naming of organic compounds?
We can easily crack the mystery of naming organic compounds if we understand the meaning of the term “organic compound.” Let’s take a closer look.
Organic Compound Naming
When it comes to naming organic compounds, there are three crucial words to remember: prefix, suffix, and root. If you can find these, you’ll be well on your way to naming an organic substance.
Naming compounds becomes hard, but we may begin by applying six stages to all compounds that need to be named. These criteria adhere to a guideline established in organic chemistry by the International Union of Pure and Applied Chemistry (IUPAC), an organisation that develops standards for naming substances. Following the IUPAC guidelines ensures that you appropriately name every organic structure.
Steps for Naming a Compound
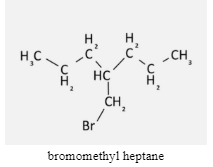
Step 1: Identify the carbon chain with the longest length in our compound.
This compound will be used as an example for naming. The longest chain, as you can see, is 7 carbon atoms long.
bromomethyl heptane
Step 2: Identify the longest carbon chain.
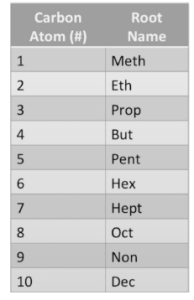
Those seven carbon atoms are the same as a root word. We can discover the root word by consulting the chart that displays how many carbon atoms correspond to which root word. The letter ‘hept-‘ relates to the number 7. You now understand that the root word is the base, which tells you how many carbon atoms are in your compound.
Step 3: Determine what the suffix (ending) should be.
If your compound has a functional group, you must identify the appropriate suffix and add it to the end of your name. A functional group is a distinct set of molecules that can be easily identified in a chemical. Because the functional group alkane (C-H atom) is present in this molecule, consult the table of suffix ends.
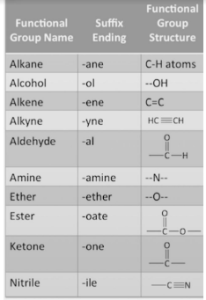
As you can see, the alkane ending is ‘-ane.’ Taking our base name of hept- and adding -ane, we get heptane as the name for the parent chain (the longest carbon-carbon chain).
Step 4: Count your carbon atoms.
First, take note of any side groups, molecules, or atoms that dangle from your longest (or parent) chain. Now, find the two ends of your carbon chain. Begin by counting each carbon atom (1,2, etc.) on the parent chain from left to right, then reverse the process. Which approach to a side group comes first? Then that end is known as the #1 carbon end.
We can see that the carbon attached to our side chain is numbered 4 regardless of which side you come from. Then 4 is the answer! As the side chain will be associated with the number 4, the number 4 will appear at the beginning of the name of our compound.
Step 5: Give the side groups names.
Circle any endpoints that are visible on the longest carbon chain. By identifying these, you will ensure that they are included in the chemical name of your compound. We have one side chain in our compound: a bromine atom connected to two carbon atoms. This is a unique chemical known as bromomethyl.
Conclusion
The basic goal of chemical nomenclature is to ensure that no misunderstanding exists between a spoken or written chemical name and the chemical compound to which the name refers: Each chemical name should only refer to one substance.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out