Free radicals are the products of normal cellular metabolism. A free radical can be defined as an atom or molecule containing one or more unpaired electrons in a valence shell or outer orbit and is capable of independent existence. The odd number of electron(s) of a free radical makes it unstable, short-lived, and highly reactive. Because of their high reactivity, they can abstract electrons from other compounds to attain stability. Thus the attacked molecule loses its electron and becomes a free radical itself, beginning a chain reaction cascade that finally damages the living cell Both ROS and RNS collectively constitute the free radicals and other nonradical reactive species.
Free radicals are highly reactive and unstable molecules that are produced in the body naturally as a byproduct of normal metabolism, or by exposure to toxins in the environment such as tobacco smoke and ultraviolet light. Free radicals have a lifespan of only a fraction of a second, but during that time can damage DNA, sometimes resulting in mutations that can lead to various diseases, including heart disease and cancer. Antioxidants in the foods we eat can neutralize the unstable molecules, reducing the risk of damage. The free radicals are produced during ATP through mitochondria. They are generally divided into two well-known entities: reactive oxygen species and reactive nitrogen species
What Are Free Radicals
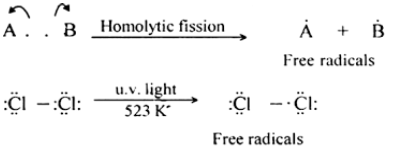
Chemical species having one or more unpaired electrons are called free radicals. Homolytic bond fission leads to the formation of free radicals. The free radicals are odd electron molecules and are highly reactive. Free radicals are paramagnetic in that they possess a small permanent magnetic moment due to the presence of unpaired electrons. This property is used for the detection of the presence of free radicals.
A notable example of a radical is the hydroxyl radical, a molecule that has one unpaired electron on the oxygen atom. The two other examples are triplet oxygen and triplet carbene (:C H2) which have two unpaired electrons.
Structure of Free Radicals
An organic free radical is a free radical form of carbon with three bonds and a single unpaired electron. A free radical can react with another free radical, but more often it reacts with a stable evenly paired molecule.
An organic free radical is a free radical form of carbon with three bonds and a single, unpaired electron. A free radical can react with another free radical, but more often it reacts with a stable, evenly paired molecule. Carbon-containing an unpaired electron in free radicals also may either be in an Sp2 hybrid state in which the structure is planar with an odd electron in the p orbital or it could be Sp3 hybridized which could make the structure pyramidal.
- Planar structure: Geometry of Alkyl halides:
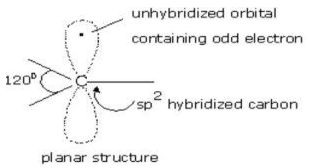
A carbon radical is sp2 hybridized with an unpaired single electron occupying an unhybridized p-orbital, having trigonal pyramidal or planar geometry possessing an angle of 120⁰.
- Pyramidal structure: SP3 hybridization, with a bond length of 109 degrees.
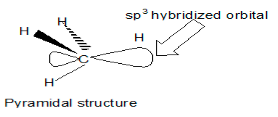
It is believed that simple free radicals have a planar structure.
As stated by Pauli, the two electrons occupying the same orbital must have opposite spins, and thus a magnetic moment of such a species becomes zero. However, free radicals have a net magnetic moment (paramagnetic) due to the presence of one or more unpaired electrons and thus can be detected by ESR. These species are highly reactive due to the presence of unpaired electrons which get paired easily with another electron to fill their outer shell. The alkyl radical has shallow pyramid geometry i.e., between sp2 and sp3 hybridization. But the energy required to invert the pyramid is very small. Practically speaking alkyl radicals are considered sp2 hybridized.
The order of stability of alkyl halides: 3 > 2 > 1.
Stability of Free Radicals
The various factors responsible for the stability of free radicals are the Inductive effect, Hyperconjugative effect, and Resonance effect.
- Inductive effect Greater the number of alkyl groups attached to the free radical carbon center will be the stability of the radical. This is due to the electron-donating inductive effect of the alkyl groups which decreases the electron deficiency of the radical.
- Hyperconjugation effect: Hyperconjugative effect also give stability to free radicals as in the case of carbocations. The stability order of alkyl free radicals is tertiary >secondary > primary > CH3. This stability order can be explained by hyperconjugation. The odd electron in the alkyl radical is delocalized onto the β-hydrogens, through hyperconjugation, which confers stability to the radical. Thus, tert-butyl radical is more stable than sec-butyl radical which in turn is more stable than n-butyl radical.
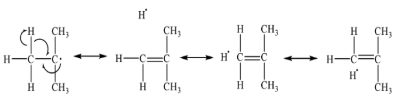
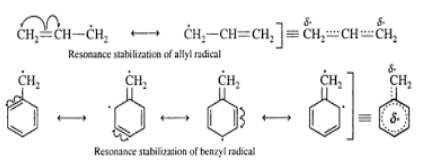
Resonance Effect: In the free radicals where the carbon center is in conjugation to a double bond, the resonance effect leads to the stabilization of these molecules. The stabilizing effects of vinyl groups (in allyl radicals) and phenyl groups (in benzyl radicals) are very significant and can be satisfactorily explained by resonance. Allyl and benzyl free radicals are more stable than alkyl free radicals but still have only a transient existence under ordinary conditions
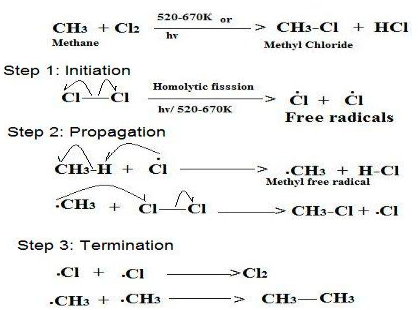
Free Radical Halogenation
In organic chemistry, free-radical halogenation is a type of halogenation. This chemical reaction is typical of alkanes and alkyl-substituted aromatics under the application of UV light. The reaction is used for the industrial synthesis of chloroform, dichloromethane, and hexachlorobutadiene. It proceeds by a free radical chain mechanism.
General Mechanism
The chain mechanism is as follows, using the chlorination of methane as a typical example:
- Initiation: splitting or homolysis of a chlorine molecule to form two chlorine atoms, initiated by UV radiation.
- Chain propagation: the hydrogen atom is pulled off the methane leaving a primary methyl radical. The methyl radical then pulls chlorine-free radicals from the chlorine atom.
- Chain termination: Recombination of two free radicals.
Conclusion
Chemical species having one or more unpaired electrons are called free radicals. Homolytic bond fission leads to the formation of free radicals. The free radicals are odd electron molecules and are highly reactive. These species are highly reactive due to the presence of unpaired electron which gets paired easily with another electron to fill their outer shell. The alkyl radical has shallow pyramid geometry i.e., between sp2 and sp3 hybridization. But the energy required to invert the pyramid is very small. Practically speaking alkyl radicals are considered sp2 hybridized
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




