An example of molecular polarizability caused by intramolecular electron displacement (also known as the ‘conjugative mechanism’ and formerly known as the “tautomeric mechanism”) is the electromeric effect. This type of polarizability is characterised by the substitution of one electron pair for another within the same atomic octet of electrons.Electromeric effect and inductive effect are frequently believed to be kinds of electron displacement.
While some refer to it as an effect caused by the presence of a reagent such as an electrophile or a nucleophile, the IUPAC does not define it that way.
The phrase “electromeric effect” has been phased out of standard textbooks and is considered outdated. The electromeric and mesomeric effects are absorbed into the phrase resonance effect. This effect can be shown using curved arrows to indicate the electron shift, as illustrated below:
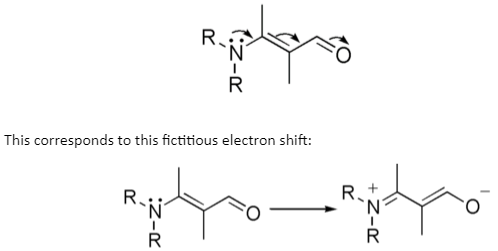
Types of Electromeric Effects
The electromeric effect is classified into two types: the +E and -E effects. This classification is based on the direction of transfer of the electron pair.
+E Effect
This phenomenon occurs when the pi bond’s electron pair is shifted toward the attacking reagent. The +E effect is visible when acid is added to alkenes. Attacking reagent binds to an atom that received an electron pair during the transfer of electrons.
A positive electron transfer (or +E effect) occurs when an electrophile attacks a positively charged atom and the pi electrons are transferred to the positively charged atom. The protonation of ethene is an illustration of the +E effect.
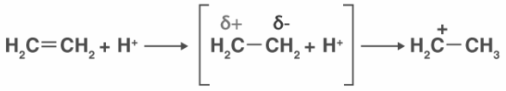
-E Effect
This phenomenon occurs when the attacking reagent’s electron pair is shifted away from the pi bond’s electron pair. The attacking reagent binds to the molecule’s positively charged atom, i.e., the atom that lost an electron pair during the transfer.
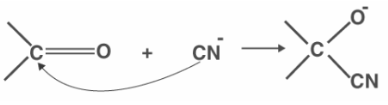
Generally, the -E effect occurs when the attacking reagent is a nucleophile and the pi electrons are moved to an atom with which the attacking reagent cannot form a connection. The -E effect occurs when nucleophiles are added to carbonyl compounds, as demonstrated below.
Examples of the Electromeric Effect
The Reaction of An Alkene With Br₂ in CCl₄
Temporary polarisation occurs as the reagent bromine approaches alkene, with the C2 atom obtaining a negative charge and the C1 atom gaining a positive charge. Alkenes are attacked by the electrophile Br+, resulting in the formation of a cyclic bromonium ion. The cyclic bromonium ion is then attacked by Br, resulting in the formation of vicinal dibromide.
Addition Of Hydrogen Halides
Hydrogen halides act as an electrophile (proton) and a nucleophile simultaneously (halide). The electrophile assaults the double bond, seizes a pair of pi electrons, and bonds them to the resultant molecule (carbocation). The reaction is completed when the nucleophile (halide) forms a new molecule.
Nucleophilic Addition Reaction
When negatively charged nucleophiles approach a carbonyl molecule, the carbonyl group becomes polarised, and the nucleophile assaults the molecule’s positive core.
Electrophilic Addition Reaction
The polarisation of the carbon-carbon double bond in symmetrical alkenes or alkynes is caused by the action of electrophiles such as H+.
Electrophilic Substitution Reactions of Benzenoids
When benzene is attacked by an electrophile, these processes result in polarisation.
Electromeric Effect Is Temporary Or Permanent
The electromeric effect is a transient one that lasts only as long as the attacking reagent is present and in contact with the organic component. When the attacking reagent is withdrawn from the system, the polarised molecule reverts to its initial state.
Electromeric Effect Is Also Known As
An example of molecular polarizability caused by intramolecular electron displacement (also known as the ‘conjugative mechanism’ and formerly known as the “tautomeric mechanism”) is the electromeric effect. Such type of polarizability is characterised by substitution of one pair of electrons for another within the same atomic octet of electrons.
Conclusion
- Inductive, electromeric, and resonance effects are three of the most frequently observed electronic effects in an organic reaction generated by the attacking reagent. Each of these three effects results in the formation of polarity in the organic substrate.
- The electromeric effect can be defined as a transient effect that creates polarity in an organic molecule with pi linked atoms.
- In contrast to the inductive effect, it is only exhibited in pi bonds and not in sigma bonds.
- Electromeric interactions are categorised as +E or -E depending on the atom with which the assaulting reagent (electrophile/nucleophile) engages.
- a +E effect is produced by an electrophilic reagent, whereas a -E effect is produced by a nucleophilic reagent.
- The protonation of ethene is an example of the +E effect, while the reaction of ketones with cyanides is an example of the -E effect.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




