Hyperconjugation is a chemical phenomenon in which the electrons of an alkyl group’s C-H bond are directly connected to an unsaturated system’s atom or an unshared p orbitals atom in an organic molecule. It is a long-lasting action that permits organic substances like glucose to be stabilised.
Definition
Includes the delocalization of (s) electrons from an alkyl group’s C-H bond when it is directly connected to an unsaturated system atom or an atom with an unshared p-orbital. The (s) electrons of the alkyl group’s C-H bond are partially conjugated with the associated unsaturated system or the unshared p-orbital. Hyperconjugation is the interaction of electrons from p systems (many bonds) with nearby s bonds (single H-C bonds) of substituent groups in organic compounds. It’s a long-term effect.
Hyperconjugation in propene is an example.
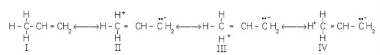
Hyperconjugation is also known as no-bond resonance because there is no link between the a-carbon atom and one of the hydrogen atoms. Although a free proton has been shown in the structures above, it is nonetheless tightly connected to the p-cloud and hence cannot travel.
Hyperconjugation order:

Applications of Hyperconjugation
Stability of Alkenes
Hyperconjugation explains why some alkenes are more stable than others.
Alkene stability is a term used to describe the ability of alkenes to remain stable throughout time. The number of alpha hydrogens is a measure of how many alpha hydrogens there are in a There are a lot of resonant structures.
Stability in decreasing order
Alkenes’ Carbon-Carbon Double Bond Size
We know that the more resonant structures there are, the more single bond character
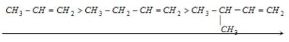
there will be in a carbon-carbon double bond.
Alkyl Carbocation Durability
Number of resonant structures µ number of alpha hydrogens alkyl carbocation stability.
Alkyl Free Radical Stability
Hyperconjugation explains the stability of alkyl free radicals. The amount of resonant structures determines stability.
R’s Ability to Release (or donate) Electrons in Alkyl Benzene
Because of the hyperconjugation, CH₃- (or alkyl group) is +R group, ortho-para directing set, and activating set for electrophilic aromatic substitution process.
The number of resonating structures, which is dependent on the amount of hydrogens present on a-carbon, determines the alkyl group’s electron donating capacity. Some
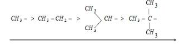
groups’ electron-releasing power is as follows:
Increasing inductive effect
Due to hyperconjugation, electrons donate power in a declining sequence.
Hydrogenation Heat
The heat of hydrogenation is reduced via hyperconjugation.
Dipole Moment
Hyperconjugation influences the dipole moment in the molecule because it impacts the formation of charges.
When the hydrogen of formaldehyde (μ= 2.72D) is replaced with a methyl group,
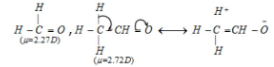
acetaldehyde (μ= 2.72D), the dipole moment increases, leading to hyperconjugation and the production of charges.
Anomeric Effect
Another type of anomeric effect is hyperconjugation, which occurs in acyclic molecules with heteroatoms. Hyperconjugation happens when an atom in a molecule has a lone pair of electrons and the nearby atom is able to accept electrons into the ∑* orbital, stabilising the molecule. This results in the formation of a “no bond” resonance form. For most heteroatoms, the trans, trans conformation is preferred for orbital overlap, but in dimethoxymethane, the gauche, gauche conformation is about 3–5 kcal/mol lower in
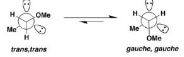
energy (more stable) than the trans , trans conformation—this is about two times as big as the effect in sugars because there are two rotatable bonds that are affected (hence trans around both bonds or gauche around both).
Synthetic Applications
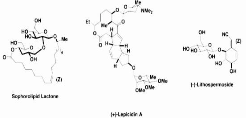
Synthetically, the anomeric effect is taken into account. Sugar and carbohydrate chemistry is one of the more prevalent synthetic uses of the anomeric effect, thanks to its discovery in sugars. The anomeric effect, for example, affects the Koenigs-Knorr glycosylation, which instals an α-OR or β-OR group in high diastereoselectivity. The compounds generated via the Koenigs-Knorr Glycosylation to overcome the anomeric effect include sophorolipid lactone, (+)-Lepicidin A, and (-)-Lithospermoside.
Gauche Effect
The gauche effect can be explained in two ways: hyperconjugation and bent links. The donation of electron density from the C–H ∑ bonding orbital to the C–F∑* an antibonding orbital is regarded as the source of stability in the gauche isomer in the hyperconjugation paradigm.
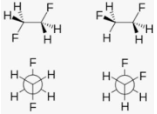
External Influences
Because of the huge polarity difference between the two conformers, the gauche effect is extremely sensitive to solvent effects. For example, 2,3-dinitro-2,3-dimethylbutane, which only exists in the gauche conformation in the solid state, favours the gauche conformer in benzene solution by a ratio of 79:21, but prefers the anti conformer by a ratio of 58:42 in carbon tetrachloride. Another example is trans-1,2 difluorocyclohexane, which prefers the di-equatorial conformer in more polar solvents rather than the anti-diaxial conformer.
Steric Hindrance
The spatial arrangement of atoms causes steric effects. The energy of the molecule increases as atoms draw closer together. Nonbonding interactions that impact the shape (conformation) and reactivity of ions and molecules are known as steric effects. Electronic effects, which determine the structure and reactivity of molecules, are complemented by steric effects. The way opposites attract and like charges repel results in organised groups of molecules stabilised by steric repulsive forces between overlapping electron clouds.
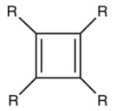
The parent cyclobutadiene (R = H)
readily dimerizes but the R = tert-butyl
derivative is robust.[
By hyperconjugation, only electrons in bonds to the positively charged carbon can stabilise a carbocation.
When the size of groups within a molecule prohibits chemical processes that occur in related smaller molecules, this is known as steric hindrance or steric resistance.
Measures of Steric Properties
Rate Data
The effects of the steric bulk of substituents can be studied using relative speeds of chemical reactions. Methyl bromide solvolysis 10⁷ times faster than neopentyl bromide under normal circumstances. The discrepancy represents the statically bulky (CH₃)₃C group’s prevention of attack on the molecule.
A- Value
The bulk of substituents can also be measured using A values. Equilibrium measurements of monosubstituted cyclohexanes provide A values. The bulk of a

substituent is determined by how much it prefers the equatorial position.
The A-value for a methyl group is 1.74 as
derived from the chemical equilibrium above.
It costs 1.74 kcal/mol for the methyl group
to adopt to the axial position compared to
the equatorial position.
Conclusion
Finally, delocalization of electron density is referred to by three terms: conjugation, hyperconjugation, and resonance (Be always careful to say that electron density is delocalized, not electrons). A better phrase may be electron dispersion. The distribution of electrons can be quite varied, resulting in significant changes in stability that are critical for understanding organic chemistry. Because, whatever else is true, the necessary orbitals are appropriately aligned and overlapped, appropriate concepts should be provided in a logical order as they relate to conjugation.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




