With the hydrogen halides, all alkenes undergo addition reactions. A few of the carbon atoms in the double bond are joined by a hydrogen atom, while the other is joined by a halogen atom.
Chloroethane can be made by combining ethene and hydrogen chloride, for example:
CH2=CH2 + HCl 🡪 CH3-CH2Cl
The addition reaction is the most prevalent chemical change of a carbon-carbon double bond. Many inorganic and organic chemicals have been discovered to add to this functional group, and we will explore many of these reactions in this section. Because the C-C pi-bond is rather weak (63 kcal/mole) compared to the sigma-bonds formed to the reagent’s atoms or groups, the bulk of these reactions are exothermic. Remember that a molecule’s bond energies are the energy required to break (homolytically) all of the molecule’s covalent bonds. As a result, the reaction will be exothermic when any bond energies of the product molecules are greater than the bond energies of the reactants.
Mechanism
The addition of hydrogen halides is one of the very easy electrophilic addition reactions since it uses the simplest electrophile: the proton. Hydrogen halides serve both an electrophile (i.e. a proton) and a nucleophile (i.e. halide). Firstly the electrophile attacks the double bond and this takes up a set of pi electrons, thereby attaching it to the molecule. This is mainly the reverse of the last step in the E1 reaction (i.e. deprotonation step). The resulting molecule will possess a single carbon- carbon bond along with a positive charge on one of them (this is referred to as carbocation). The next step is seen when the nucleophile (i.e. halide) bonds to the carbocation, leading to the formation of a new molecule with both the original hydrogen and halide attached to the organic reactant. The second step will proceed only if a good nucleophile is used.
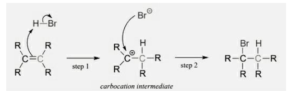
In this reaction, all halides (HBr, HCl, HI, HF) can participate and add on in the same way. Although different halides react at different rates, this is owing to the weakening of the H-X bond as X grows larger (low orbital overlap).
Rearrangement of Carbocation
Carbocation development is occasionally followed by structural reorganisation. Such rearrangements are favoured when the rearranged carbocation is more stable than the initial cation, and they occur by shifting a nearby alkyl group or hydrogen. When HCl is added to 3,3-dimethyl-1-butene, for example, an unexpected product, 2-chloro-2,3-dimethylbutane, is produced in slightly higher yield than the expected Markovnikov product, 3-chloro-2,2-dimethylbutane. This unexpected outcome could be explained by a 1,2-shift of a methyl group causing a carbocation rearrangement of the previously produced 2°-carbocation to a 3°-carbocation.
Strain is another condition that might cause carbocation intermediates to rearrange. The rearrangement product, bornyl chloride, is produced in great yield when HCl is added to -pinene, the primary hydrocarbon component of turpentine. In the equation, this rearrangement changes a 3°-carbocation to a 2°-carbocation, which is a generally unfavourable transition. The rearrangement, on the other hand, stretches a stretched four-membered ring into a significantly less strained five-membered ring, providing a driving force for the rearrangement.
Reaction rates
Variation of rates when halogen is changed
Reaction rates increase in the order HF < HCl < HBr < HI. Because hydrogen fluoride interacts much more slowly than the other three, it is sometimes overlooked when discussing these interactions.
The hydrogen-halogen link must be broken when hydrogen halides react with alkenes. As you move from HF to HI, the bond strength drops, and the hydrogen-fluorine bond is especially strong. Because it’s difficult to break the link between hydrogen and fluorine, adding HF will take a long time.
Variation of rates when the alkene is changed
This is true for both symmetrical and asymmetrical alkenes. The examples below are all symmetrical for simplicity’s sake, but they don’t have to be. As the alkene becomes more intricate (in terms of the number of alkyl groups (such as methyl groups) connected to the carbon atoms at each end of the double bond), reaction rates increase. Consider the following scenario:
 There are two approaches to look at the causes for this, both of which require knowledge of the reaction process. Since electrons in the π bond attract anything with a positive charge, alkenes react. This will be aided by anything that boosts the electron density surrounding the double bond. Alkyl groups have a proclivity for “pushing” electrons away from themselves and toward the double bond. The region around the double bonds grows increasingly negative as the number of alkyl groups increases. The more negatively charged the region grows, the more molecules like hydrogen chloride will be drawn to it. The stability of the intermediate ion generated during the reaction is the most essential cause. The results of the three cases presented above produces (carbonium ions) at the half-way stage of the reaction:
There are two approaches to look at the causes for this, both of which require knowledge of the reaction process. Since electrons in the π bond attract anything with a positive charge, alkenes react. This will be aided by anything that boosts the electron density surrounding the double bond. Alkyl groups have a proclivity for “pushing” electrons away from themselves and toward the double bond. The region around the double bonds grows increasingly negative as the number of alkyl groups increases. The more negatively charged the region grows, the more molecules like hydrogen chloride will be drawn to it. The stability of the intermediate ion generated during the reaction is the most essential cause. The results of the three cases presented above produces (carbonium ions) at the half-way stage of the reaction:
 The stability of the intermediate ion generated during the reaction is the most essential cause. At the half-way point of the reaction, the three instances above yield these carbocations (carbonium ions):
The stability of the intermediate ion generated during the reaction is the most essential cause. At the half-way point of the reaction, the three instances above yield these carbocations (carbonium ions):
Conclusion
Alkenes are generally unsaturated hydrocarbons, which means that each molecule possesses at least one double bond. They demonstrate addition reactions in which an electrophile assaults the carbon-carbon double bond to create addition products due to the presence of pi electrons. Electrophilic addition reactions of alkenes are the name for these reactions. These addition reactions might also follow a free radical mechanism. Alkenes undergo a variety of reactions, these includes both oxidation and ozonolysis.
Electrophilic addition reactions can be seen in a wide range of alkenes. Electrophilic addition reactions of alkenes comprises the addition of hydrogen halides like those of hydrogen chloride and hydrogen bromide. HI >HBr> HCl is the usual tendency for hydrogen halide. In comparison to unsymmetrical alkenes like propene, it is much easier to forecast the end result for symmetrical alkenes like ethene.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




