Brady’s reagent is a chemical compound. 2. 4-dinitrophenylhydrazine (Brady’s reagent) is a reddish-orange solid that is usually supplied wet to reduce the risk of explosion.
Chemical formula: C6H6N4O4
Synthesis of 2,4-dinitrophenylhyadrazine:
Despite the fact that hydrazine H2N-NH2 is not a commonly substituted hydrazine, we can draw a reasonable conclusion that it is a good nucleophile based on its structure. To make 2,4-Dinitrophenylhydrazine from the product, which is prepared by reacting hydrazine with 2,4-dinitrochlorobenzene, the product must first be converted to hydrazine. Because of the electron-accepting properties of the two nitro groups, this chloride is relatively easy to displace.
We should nitrate chlorobenzene because chlorine is ortho, para, and deactivating, and thus we should nitrate chlorobenzene. Because chlorobenzene can be readily synthesised from benzene, the synthesis is complete, and the reaction is described in detail below.
Brady’s reagent:
Brady’s reagent is an aqueous solution of 2,4-dinitrophenyl hydrazine (DNP), which is used in the analysis of DNA. A coloured precipitate is formed when it reacts with carbonyl compounds (aldehydes and ketone) in the presence of water. The melting point of these precipitates is extremely high. The melting points of the precipitates confirm that they are composed of carbonyl substances.
Benzoic acid is used as an antiseptic in medicines for urinary disorders, and it is also used in vapour form to disinfect the bronchial tubes.
Acetic anhydride reacts with N2O5 to form acetyl nitrate, which is a poisonous gas.
The reaction:
(CH3CO)2O + N2O5 → 2CH3COONO2 (Acetyl nitrate)
In alkyl groups, the amount of acid chloride that reacts with water decreases as the number of C-atoms in the group increases.
CH3COCl > CH3-CH2-COCl > CH3-CH2-CH2COCl > ………
Identifying a carbonyl compound:
The carbonyl compound is simply mixed with an acid solution of Brady’s reagent in methanol to produce the desired result.. The derivatives are crystalline solids with an orange colour that are known as 2,4-dinitrophenylhydrazones. These crystals are filtered out and purified through a process known as re-crystallisation.
The melting temperatures of these materials are measured. The original carbonyl compound is then identified by comparing its melting point to the melting points of 2,4-dinitrophenylhydrazine derivatives.
Because it produces orange crystals when added to either an aldehyde or a ketone, Brady’s reagent can be used as a test for the presence of carbonyl compounds as well.
2,4-Dinitrophenylhydrazine Reaction with Ethanal:
2,4-Dinitrophenylhydrazine reacts with both aldehydes and ketones to form a 2,4-dinitrophenylhydrazone, which is a reactive intermediate in the reaction. As an illustration, consider ethanal.
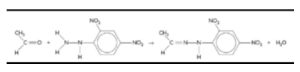
- It is a condensation reaction that is taking place (water is eliminated).2,4-dinitrophenylhydrazone (ethanal 2,4-dinitrophenylhydrazone) is the name given to the product after the name of the aldehyde or ketone is followed by 2,4-dinitrophenylhydrazone.
- 2,4-dinitrophenylhydrazine can be used as a method for identifying aldehydes and ketones because the 2,4-dinitrophenylhydrazone that is formed can be purified and its melting point determined. A comparison of the melting point with a table of known values could be used to identify the aldehyde or ketone in question.
- All of the 2,4-dinitrophenylhydrazone derivatives are solids in the orange/yellow colour spectrum.
- Brady’s reagent is a term used to refer to a solution of 2,4-dinitrophenylhydrazine in water.
Conclusion:
2,4-Dinitrophenylhydrazine is the chemical compound C6H3(NO2)2NHNH2 that is used to make it. Dinitrophenylhydrazine is a solid that ranges in colour from red to orange. It is a hydrazine that has been substituted. The solid is sensitive to shock and friction in comparison to other materials.
Brady’s reagent is an aqueous solution of 2,4-dinitrophenyl hydrazine (DNP), which is used in the analysis of DNA. A coloured precipitate is formed when it reacts with carbonyl compounds (aldehydes and ketone) in the presence of water.Acetic anhydride reacts with N2O5 to form acetyl nitrate, which is a poisonous gas.
The carbonyl compound is simply mixed with an acid solution of Brady’s reagent in methanol to produce the desired result.. The derivatives are crystalline solids with an orange colour that are known as 2,4-dinitrophenylhydrazones. These crystals are filtered out and purified through a process known as re-crystallisation.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




