Inhibitors, both reversible and irreversible, attach to an enzyme and decrease its activity. One way to do this is to bind to an enzyme for an extended period. These inhibitors are known as irreversible inhibitors. Other substances, on the other hand, can temporarily bind to an enzyme. These are said to as reversible. Competitive inhibitors bind to an active site, while reversible inhibitors bind to another site on the enzyme (non-competitive inhibitors).
What is Irreversible Inhibition?
Irreversible inhibitors bind to an enzyme covalently, making this sort of inhibition difficult to reverse. Nitrogen mustards, aldehydes, haloalkanes, alkenes, Michael acceptors, phenyl sulfonates, and fluorophosphate are common reactive functional groups found in irreversible inhibitors. Covalent adducts are formed when these nucleophilic groups react with amino acid side chains. The amino acids serine (as in DFP, right), cysteine, threonine, or tyrosine are among the residues that include side chains containing nucleophiles such as hydroxyl or sulfhydryl groups.
Irreversible inhibition is not the same as irreversible inactivation of an enzyme. Irreversible inhibitors are enzyme inhibitors that are selective for one class of enzyme and do not inactivate all proteins; they work by modifying the active site of their target rather than damaging protein structure. Extremes in pH or temperature, for example, typically promote denaturation of all protein structures, but this is a non-specific effect. Similarly, several non-specific chemical treatments degrade protein structure: for example, heating proteins in concentrated hydrochloric acid hydrolyzes peptide bonds, releasing free amino acids.
Types of Irreversible Inhibitors:
Group-Specific Reagents: Diisopropylphosphofluoridate (DIPF) and iodoacetamide are examples of group-specific reagents that react with certain amino acid side chains. DIPF, for example, modifies only one of the 28 serine residues in chymotrypsin. This indicates that this particular residue is very reactive; also, it is assumed that this particular residue is located in the active site of the enzyme chymotrypsin.
Affinity Labels: Affinity labels (Reactive substrate analogues) are more particular than group-specific reagents since they are structurally identical to the substrate that can covalently bind to the active site. Tosyl-L-phenylalanine chloromethyl ketone (TPCK) is an analogue for chymotrypsin that binds to the active site and inhibits the enzyme irreversibly by reacting with the histidine residue.
Suicide inhibitors: They (Mechanism-based inhibitors) attach to the enzyme as a substrate, which is then processed by a normal catalytic mechanism, which produces a chemically reactive intermediate, which inactivates the enzyme by covalent modification. The inhibitory power of the planar proline intermediate created during proline racemization is an example of a mechanism-based inhibitor. A trigonal intermediate is generated during this process, and the development of the racemase is hindered because the tetrahedral intermediate required for product formation is not formed. Proline isomerization via the planar transition state emphasises the importance of transition-state analogues as enzyme inhibitors.
Measurement :
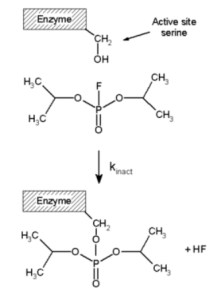
Irreversible inhibitors build a reversible non-covalent complex with the enzyme (EI or ESI), which then reacts to produce the covalently modified “dead-end complex” EI*, as indicated in the diagram to the right (an irreversible covalent complex). The inactivation rate, or kinase, is the rate at which EI* is produced. Because the production of EI may compete with the creation of ES, irreversible inhibitors can be prevented from binding by competing with the substrate or a second, reversible inhibitor. This protective effect is strong evidence for an irreversible inhibitor’s particular interaction with the active site.
By incubating the enzyme with an inhibitor and measuring the amount of activity leftover time, the binding and inactivation steps of this reaction are examined. The activity will gradually decrease over time, usually following an exponential decline. The rate of inactivation at this concentration of inhibitor is calculated by fitting these data to a rate equation. This is done at numerous different inhibitor doses. The inactivation rate will be saturable if a reversible EI complex is involved, and fitting this curve will give kinact and Ki.
Other Cases :
Irreversible inhibitors do not always create covalent adducts with the enzymes they target. Some reversible inhibitors attach to their target enzyme so tightly that they become irreversible. The kinetics of these tight-binding inhibitors may be similar to those of covalent irreversible inhibitors. Some of these inhibitors attach to the enzyme quickly in a low-affinity EI complex, which then undergoes a delayed rearrangement to a highly tightly bound EI* complex in certain circumstances. Slow-binding is the name for this type of kinetic behaviour. As the enzyme “clamps down” around the inhibitor molecule, this gradual rearrangement generally entails a conformational shift. Some critical medications, such as methotrexate, allopurinol, and the activated version of acyclovir, are slow-binding inhibitors.
Conclusion
The elimination of the excess inhibitor from the system will not be able to reverse irreversible inhibition. The elimination of the inhibitor from the system is required for reversible inhibition recovery, but the synthesis of the new enzyme is required for irreversible inhibition recovery. A reactive grouping is connected to a substrate analogue in active-site-directed inhibitors. Because mechanism-based inhibitors rely on the enzyme’s activity to produce a reactive intermediate that then reacts irreversibly with the enzyme, they can be quite selective. In divergent processes, suicide substrates act as both substrates and irreversible enzyme inhibitors.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




