The photoelectric effect is a phenomenon where the energy dissipated from the light causes electrons to be released from the metal’s surface. Photoelectrons are the electrons that are discharged. The frequency of the incident light coming from a source reflecting on the metal’s surface affects the rate of emission of photoelectrons and the kinetic energy of the emitted photoelectrons. The photoelectric effect happens when electrons from the metal’s surface absorb the energy from incident light and then use it to counteract the attraction forces that attach them to the nuclei of the metal.
History Of Photoelectric Effect
In 1887, Wilhelm Ludwig Franz Hallwachs first described the discovery of the photoelectric effect, another scientist Heinrich Rudolf Hertz dealt with the experimental kind of stuff verification. Both of these scientists discovered that when we subject a metal surface to any electromagnetic radiation which is high in frequency, the absorption of energy starts taking place, which results in the emission of electrons. The photoelectric effect is a process in which a material( usually a metal or semiconductor) sinks in complete electromagnetic radiation which results in the dissipation of charged items like photons.
More specifically, the photoelectric effect causes electrons present inside the atoms of that metal atom to rise up different shell levels to be discharged when the light source is focused on a metal surface. A photoelectron can also be termed as an electron that has been dejected out as a final result from the metal’s surface of the photoelectric effect and is symbolised by the e- (e negative).
Photons & Photoelectric Effect
The Photoelectric effect works and relies upon the particle nature of light; it works on the principle of wave nature of light. When a photon makes contact with an electron, some energy is used to eject the electron.
A photon is a little ball of radiant energy. A wandering blob of energy attacks the electron in the photoelectric effect. As a result, the electron becomes agitated, breaking its bond with the parent atom to which it is attached. If the conditions are right, we can employ light to nudge electrons away from a solid’s surface.
The energy of one photon is directly proportional to the frequency of light.
E = h𝜈 = hc/λ
where,
E = energy of a unit photon
h = Planck’s Constant (6.62607004 × 10-34 m2 kg / s)
𝜈 = frequency of the light source
c = speed of light(vacuum)
λ = wavelength of light source
Threshold Frequency
By the name only, we can guess what is threshold frequency?. Threshold means the level at which something or some process starts to occur. Similarly, threshold frequency means the frequency at which the photons that hit the metal or a semiconductor should have ample energy to break the bond between the electrons with their nuclei because the force of attraction that connects has to be broken for the photoelectric effect to occur.
The threshold energy(also called the work function) is represented by the symbol Φ. Threshold energy is derived from the related term threshold frequency. Threshold Frequency is the minimum amount of energy that is needed by a free electron for it to absorb the energy and excite to higher shells and finally break out from the metal top surface. The frequency of any photon should be equal to the threshold frequency, for the energy dissipated to have the same energy as the threshold energy.
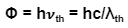
Frequency of the Photon & Kinetic Energy of the Photoelectron

For the photoelectric effect to take place there are certain conditions that have to be met in regards to threshold frequency. If the photon with energy is less than the threshold energy of that material, then no emission of photoelectrons can take place because the cohesive intramolecular forces between the electrons and the nucleus cannot be overcome. The photoelectric effect cannot take place if 𝜈 < 𝜈th. A case arises here, what if the frequency of the photon injected over the metal surface is the same as the threshold frequency of the metal (𝜈 = 𝜈th), the photoelectrons will receive only that much energy with the help of which they can come up to the metal surface but can’t have motion because the kinetic energy will be equal to zero.
Different metals have different threshold energies because the attractive forces that connect electrons to metals differ depending on the metal. It should also be mentioned that the photoelectric effect can occur in non-metallic substances, but their threshold frequencies are typically very high.
Conclusion
Photoelectric effects have multiple applications such as generation of electricity from solar panels, burglar alarms, photomultipliers, video camera tubes, and developing night vision spectacles. The photoelectric effect is used in motion sensors. The photoelectric effect occurs when light strikes a metal’s surface and causes electrons to be discharged. The quantity of electrons emitted is affected by the intensity, and the kinetic energy of the ejected electrons is affected by the frequency. The intensity of incident radiation is directly proportional to the magnitude of photoelectric current.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




