The majority of natural and artificial reactions take a long time to get completed. A material known as a catalyst must be applied to speed up the process. As a result, catalysis is the procedure of expediting a reaction. The catalyst in catalysed mechanisms reacts typically to make a transient intermediate, which would be subsequently used to replenish the original catalyst together in a cyclic process.
What is Catalysis?
Catalysis involves a material that increases the pace of a chemical reaction and which is not consumed during the catalysed reaction, and, therefore, can function multiple times. Even a tiny amount of a catalyst is often enough to achieve this effect.
Mechanism of Catalysis
Although less energy is necessary to accomplish the transition state in the presence of catalysts, the total free energy from the reactants to the products remains the same. A catalyst can be used in a range of chemical reactions. The inclusion of other compounds known as inhibitors (which limit catalytic activity) and promoters can change the impact of a catalyst.
At the same temperature for the same concentration of reactants, catalysed reactions have lower activation energy (rate-limiting free energy of activation) over uncatalysed reactions, resulting in a faster reaction rate. The rate of a reaction, like any other chemical reaction, is determined by the frequency with which the reactants come in contact in the rate-determining phase.
Typically, a catalyst is involved in the slowest stage. The rate of reaction is determined by the amount of the catalyst used. While catalysts are still not consumed during the reaction, later reactions can restrict, deactivate, and eliminate them.
Types of Catalysis
A catalyst is a material that speeds up the rate of a process without being absorbed by it. Catalysis refers to any reaction that uses a catalyst. When reading chemistry study material, remember that a catalyst (plural “catalysts”) is a physical entity, but catalysis (plural “catalyses”) is a process.
Homogeneous Catalysis
The reaction is homogeneously catalysed when the catalyst and the reactant(s) are all in the same physical state or phase but most often in the liquid phase. This is particularly common with gaseous catalyst-reactant combinations. Organic acids wherein the given hydrogen atoms are replaced by a metal, various molecules including carbon or metal components in some form, and carbonyl compounds connected to cobalt or iron are examples of homogeneous catalysts.
The transformation of persulphate plus iodide ions to sulphate ion but also iodine is an example of a sort of liquid catalysis:
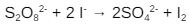
Since both reactants are negatively charged, their electrostatic characteristics contradict their chemical qualities. Such a reaction will have a tough time progressing on its own with the favourable energetics. When positive-charged iron ions are introduced to the solution, the iron “distracts” the negative charges, allowing the reaction to proceed swiftly.
The conversion of oxygen gas, O2, in the atmosphere into ozone, O3, is a naturally found gaseous homogeneous catalysis In which oxygen radicals (O-) constitute intermediates. The actual catalyst is ultraviolet light from the sun, even though every physical chemical is in the same (gas) condition.
Heterogeneous Catalysis
When the catalyst and also the reactant(s) are all in distinct phases and the reaction proceeds at the interfaces among them, the reaction is said to be heterogeneously catalysed (most commonly, the gas-solid “border”). Inorganic – non-carbon-containing – solids like primary metals, sulphides, and metallic salts, and even a smattering of other organic compounds like hydroperoxides or ion exchangers are some of the more prevalent heterogeneous catalysts.
Zeolites are a type of heterogeneous catalyst that is widely used. Such crystalline solids are made of SiO4 repeating units. Various rings and cage structures have been formed by linking four of these joined molecules together. A charge imbalance is generated in the crystal either by the existence of an aluminium atom, which is balanced by a proton (i.e., a hydrogen ion).
Autocatalysis
Autocatalysis is the catalysis of a process by one or more of its products. Autocatalysis has been the basis of modern biogenetic ideas, where they have been focused on metabolism, genetic replicators or confinement reproducers. It can be considered a minimum need for the formation of life. Autocatalysis has been one of the methods for shattering chiral symmetry in chemical reactions and production of patterns or ordered periodic behaviour.
Photocatalysis
Photocatalysis is a reaction in which a catalyst receives light (such as visible light), gets stimulated, but then conducts an intersystem crossing with a starting material before returning to the ground state without even being consumed. The excited state of the initial material would then undergo normal reactions. It couldn’t be adequately illuminated. Photocatalysis, for instance, is commonly used to make singlet oxygen. Photocatalysts can also be used in dye-sensitised solar cells as a significant component.
Conclusion
Catalysts are chemicals that change the rate of a chemical reaction by changing the process’s path. A catalyst is usually used to speed up or raise the rate of a reaction. Catalysts are also utilised to break or reestablish chemical bonds among atoms in molecules of various elements and compounds. Catalysts, in essence, stimulate molecules to interact, making the entire reaction mechanism easier and quicker.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






