Soap and detergent are compounds that may remove dirt from surfaces such as human skin, fabrics, and other solids when dissolved in water. If oil droplets and dirt particles were not suspended in the detergent solution in a stable and widely dispersed state, they were more likely to flocculate, or consolidate into aggregates large enough to reappear on the cleansed surface.
SOAPS
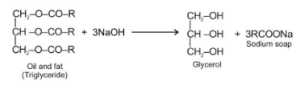
Saponification :
Chemically, oils and fats are triesters of glycerol and fatty acids. As a result, they’re known as triglycerides or triacylglycerol. When a caustic alkali is used to hydrolyze them, they produce glycerol and soaps.
Soap with eight to eighteen carbon atoms is of good quality. Water solubility reduces as the number of carbon atoms increases, but cleansing power diminishes as the number of carbon atoms drops.
Methods of Preparation of Soaps :
Soap can be prepared by the following methods.
Cold Process: in this process involves the combining some oils like olive, coconut, and palm with an alkali (sodium hydroxide) as a result, produce a bar of soap known a saphonification.
Hot Process: In this process, an external heat source is used to get the soap to the gel phase, after which it is poured into the mould.
DETERGENTS
Detergents, also known as synthetic scavengers, are purifying reagents that resemble soap but are not soap. Synthetic detergents are prepared goods that comprise alkyl hydrogen sulphate sodium salts or alkyl benzene sulphonic acid sodium salts. Detergents are superior than soaps because they may be used in both hard and acidic water. Detergents work in a similar way to soaps when it comes to cleaning.
When the water is rinsing away, the oil is rinsing away as well. As a result, the dirt is removed from the fabric, and the water is used to wash away the droplets. Because the micelles’ surfaces are all negatively charged and resist each other, they do not form big droplets. Soap’s capacity to clean is based on its ability to function as an emulsifier between water and water-insoluble greases.
There are three types of detergents.
1. Anionic Detergent
Alkyl hydrogen sulphates are generated by reacting long-chain alcohols with concentrated sulfuric acid, and when neutralised with alkali, they become neutral detergents.
Anionic detergents such as sodium salts of alkyl benzene sulfonates are common.
They’re generally used in the household. – Anionic scavengers can also be found in dentistry.
2. Cationic Scavengers
Quiescent salts produced with acetate, chloride, or bromide anions of amines are known as cationic scavengers. The cationic component has lengthy hydrocarbon chains and the nitrogen molecule has a positive charge. As a result, they’re known as cationic detergents.
3. Non-ionic Scavengers
Non-scavengers have no ions in their structure. The interaction of stearic acid and polyethene glycol produces one such detergent.
Anaerobic liquid scavengers are used to clean dishes.
The main issue with synthetic detergents is that bacteria cannot easily breakdown them if they have more branched hydrocarbon chains.
They persist in the water after sewage treatment, producing foam in river ponds and springs, polluting the water.
Difference between Soaps and Detergents :
Soaps and detergents are sodium or potassium salts of higher monocarboxylic aliphatic acids, whereas soaps are sodium salts of higher alkane sulphonic acids of alkane hydrogen sulphates. As a result, the polar heads of soaps and detergents have a chemical difference.
The second difference is that soaps are the salts of (which are weaker acids) and
(which is a strong base), whereas detergents are the salts of stronger acid
or
and strong base,
. This is the reason why the
or
ions present in hard water form insoluble calcium and magnesium salts reacting with soap because these are basically covalent in nature.
As a result, soaps initially produce calcium and magnesium soap precipitates. Soaps provide only lather once all the Ca and Mg ions have been eliminated. Because detergents’ Ca and Mg salts are ionic in nature and hence soluble in water, they produce lather even in hard water.
Soaps produce alkaline water solutions owing to hydrolysis, whereas detergent aqueous solutions are neutral. This is why detergents, rather than soaps, are used to wash woollen silk and other delicate fabrics.
About Surfactants
Surfactants enhance cleaning by lowering surface tension and allowing water to flow uniformly across the surface. This results in more uniform dampness, making it easier to wipe away and eliminate dirt and soil. Surfactant molecules can be positive or negative in charge, with one end attracted to water and the other to dirt and grease. This allows detergents to attach to dirt, break it up, and wash it away with water.
Behaviour in Water
Detergents make up the majority of cleaning products today. The way soap reacts with water is one of the main reasons behind this. While detergents are free-rinsing (i.e., they don’t leave a residue), soap requires a clear water wash after use to avoid leaving a film.
Soap is harmed by hard water. Soaps produce scum when they come into contact with harsh water. Soap scum has a negative impact on fabrics, causing them to degrade and eventually destroy garments or other surfaces. Finally, soaps require warm water to function. Detergents, on the other hand, can be designed to work in any temperature of the water versatility.
Conclusion:
When dissolved in water, soap and detergent can remove dirt from surfaces such as human skin, fabrics, and other substances. Oil droplets and dirt particles that were not suspended in a stable and widely dispersed form in the detergent solution were more likely to flocculate, or congeal into aggregates large enough to resurface on the cleansed surface. Detergents, also referred to as synthetic scavengers, are purifying reagents that seem like soap but aren’t. Synthetic detergents are made up of sodium salts of alkyl hydrogen sulphate or sodium salts of alkyl benzene sulphonic acid.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out