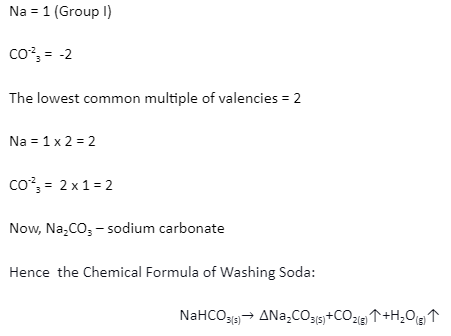A chemical equation is a way of representing a chemical reaction. The reactants refer to the substances that initiate the chemical reaction. They are written on the left side of the equation. The products are the substances that emerge from the chemical reaction. They are represented on the right side of the equation. The coefficients adjacent to the entity symbols refer to the number of moles of a substance produced or consumed during the chemical reaction.
Rules for Writing Chemical Equations
The reactants are written on the left side of a chemical equation, and the products are written on the right. The coefficients adjacent to the entity symbols represent the number of moles of a substance produced or consumed during the chemical reaction. If there are two or more reactants and products, they should be separated by a ‘+’ sign.
A chemical equation is made up of the chemical formulae of the reactants (on the left) and the products (on the right). An arrow symbol ” →”, which is commonly referenced as “yields” separates the two sides. A “+” sign separates the chemical formulas of each particular substance from those of others. Each compound’s or molecule’s state of matter is given in subscript next to the compound by an abbreviation in parenthesis.
A compound in the gas form, for example, would be denoted by (g), solid (s), liquid (l), and aqueous (a) or (aq). Aqueous implies dissolved in water and refers to the condition of matter of acids, bases, and dissolved ionic compounds.
The following Rules for Writing Chemical Equations must be observed while creating chemical equations:
- A chemical equation should be written with the reactants on the left side of an arrow and the products of the chemical reaction on the right side of the equation.
- The arrow’s head should always go to the right or the products’ side of the equation. Sometimes the arrow indicates equilibrium with the reaction proceeding in both directions simultaneously.
- At the top of the arrow, write the conditions needed for the reaction to proceed (for example, temperature, pressure, catalyst, etc.).
- By writing the state inside the bracket next to the reactants and product, the physical properties of the reactants and products should be communicated. For gas, write O2(g), same for liquids (l), solids (s), aqueous solutions (aq), and so on.
- Chemical equations must be balanced using the law of conservation of mass, which specifies that the number of atoms of each element on the reactants side should match the number of atoms with the same elements on the products’ side. For example, a balanced chemical equation with applications of the above rules is as follows:
6CO2 + 6H2O → C6H12O6 + 6O2
This is the chemical equation for photosynthesis. Photosynthesis occurs in plants in the mesophyll of the leaves, within the chloroplasts. Chloroplasts contain thylakoids, which are disc-shaped structures storing the pigment chlorophyll.
Chemical Formula Writing Standards
To write the chemical formula of a binary combination, the following requirements must be followed: one must know the valencies of the two components or radicals present. The sum of the entire number of sample valencies present in a molecule should be zero in a chemical formula. This can be accomplished by calculating the lowest common multiple of the two valencies. If the metal is present, it must always be placed at the beginning of the formula.
Conclusion
With this, we come to an end to the Rules for Writing Chemical Equations. When you study chemistry, Rules for Writing Chemical Equations is one of the most basic concepts.The Rules for Writing Chemical Equations is first to write the symbols with positive charge valency. Next, write the valency of each atom at the top of its symbol. Finally, split the valency number by their highest common factor, ignoring the positive or negative radicals. The radical’s valency should be switched. Finally, write the interchanged valency number to the radical’s lower right.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out