The laws of chemical combinations created beat back in the stagnant pole of chemistry. They sanctioned scientists to carry out various experiments that help in forming strong foundations in chemistry. However, proof and experimental evidence are still required for these laws. It was around 1801-1810 that a physicist and a chemist from England named John Dalton was successful in answering these questions. A theory was proposed by him known as Dalton’s Atomic Theory.
Dalton’s Atomic Theory
With the theory proposed by John Dalton many concepts regarding matter, atoms, the composition of matter, and even the combination of atoms resulting in compounds is better understood. On the law of constant proportion and the law of conservation of mass, Dalton grounded his theory. There are 6 postulates proposed by Dalton in his theory known as Postulates of Dalton’s Atomic Theory.
Postulates of Dalton’s Atomic Theory
The Postulates of Dalton’s Atomic Theory is as follows:-
Dalton’s Atomic Theory includes 6 postulates –
- In a chemical reaction atoms cannot be divided, i.e. is neither created nor destroyed
A new unit is formed in a chemical reaction by the combination of atoms. Neither the existing atom can be destroyed nor is a new atom formed. Only the arrangements of the atom change in the chemical reaction to form a unit, no new atoms are formed, so atoms are fundamental units. For example,
2H2 + O2 → 2H2O
Therefore, if 2H2 is considered as element ‘A’ and O2 as element ‘B’ then 2H2O is a compound formed by just changing the arrangement without forming any new atom.
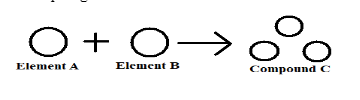
- Very tiny particle known as atoms together forms matters that participate in a chemical reaction –
For example, the Tip of a pencil is made up of carbon atoms and also gold ornaments are made up of gold atoms. Like element ‘A’ and element ‘B’ together form compound ‘C’.
- The atoms of the same element have the same mass and similar chemical properties always –
O2 → O + O
Both the atoms have the same mass i.e. 16µ and the same chemical properties.
- To form compound atoms combine in the small whole-number ratio –
The ratio combinations of atoms are always in the whole number.
For example,
CO2 =1:2, i.e. CO2 contains one atom of carbon and 2 atoms of oxygen. Therefore the ratios are in the whole number and not in numbers like ½ or ¾ etc.
- There are different masses and different chemical properties in atoms of a different element.
For example,
HCl → H + Cl
As there are two different atoms hydrogen and chlorine. So both the atoms have different masses and different chemical properties.
- In a given compound the relative numbers and the kinds of atoms will be constant.
For example,
CO2 → C + O2
Therefore, the compound contains one carbon atom and two oxygen atoms and it remains the same always.
Drawbacks of Dalton’s Atomic Theory
Drawbacks of Dalton’s Atomic Theory are as follows:-
- According to Dalton’s Atomic Theory, to form a compound, atoms combine in the small whole-number ratio. But this is not always true because in some complex organic compounds such as sugar C12H22O11 the ratio changes.
- According to Dalton’s Atomic Theory, in a chemical reaction atoms cannot be divided, i.e. are neither created nor destroyed. But this was proved wrong atoms can be further divided into protons, neutrons, and electrons.
- According to Dalton’s Atomic Theory, there are different masses and different chemical properties in atoms of a different element. But this was proved wrong in some cases because the calcium, as well as argon atoms, have an atomic mass of 40 AMU. These types of atoms are called isobars.
- According to Dalton’s Atomic Theory, the atoms of the same element always have the same mass and similar chemical properties. But this was proved wrong because chlorine is an atom that has two different isotopes which have mass numbers of 35 and 37.
Conclusion
It is to conclude that with the theory proposed by John Dalton many concepts regarding matter, atoms, the composition of matter, and even the combination of atoms resulting in compounds is better understood. There are 6 Postulates of Dalton’s Atomic Theory which also have several drawbacks or limitations.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






