In 1896, a German chemist called Wilhelm Ostwald was the first to develop the term “mole.” The term “mole” comes from a Latin word that means “a pile.” A mole was formerly defined as the element carbon.
It was defined as the amount of stuff containing the same number of fundamental entities as the number of atoms in a 12g pure carbon sample. However, this definition has been changed, and one mole is now equal to Avogadro’s constant value. 6.022 x 1023 entities are included in one mole. This number is known as Avogadro’s number; it is a numerical constant that may represent atoms, ions, or molecules.
Mole Concept
A mole is a measurement unit for the number of fundamental entities (atoms, molecules, and ions) present in a material. The idea of a mole is related to that of weight in that both help determine the amount of material present.
On a macroscopic level, the mole concept allows us to put quantitative information about what happens in a chemical equation. Two moles of water are degraded into two moles of molecular hydrogen and one mole of molecular oxygen in the chemical reaction 2H2O + O2 → 2H2.
Suppose beaker A contains 10 moles of water (a chemical compound), and beaker B contains 10 moles of mercury (a chemical element). In that case, the two beakers contain equal amounts of the substance. Beaker B contains precisely one atom of mercury for each water molecule in beaker A, even though the two beakers have different volumes and masses of liquid.
A recent event on the Mole concept
On the 20th of May, 2019, these adjustments went into effect. Following these adjustments, a substance’s “one mole” was redefined as containing “exactly 6.02214076 X 1023 elementary entities.”
Formula
For the number of Moles of molecules:
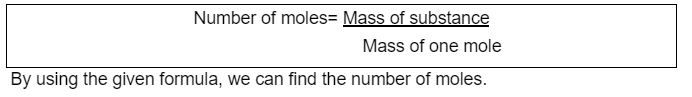
Here the mass of one mole is also called Molar mass. The mass of one mole of a pure substance is the molar mass of that substance which is taken in grams.
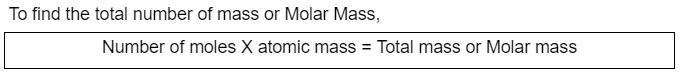
Water, for example, has a molar mass of 18.015 g/mol, which is equal to the mass of the NA number of water molecules.
Let’s consider SO3
Here, 1 mole S X 32.1g = 32.1g S
3 mole O X 16.0g = 48.0g O
Molar mass of SO3 is 80.1g
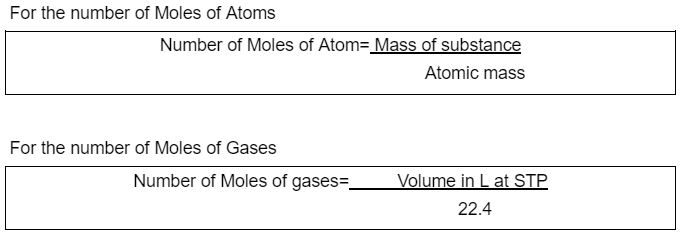
For the number of Moles of Particles
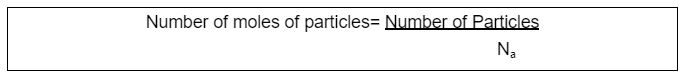
Numericals on mole concept
What is the mass (in grams) of one carbon atom if one mole of carbon atoms weighs 12 grams?
Given: Mass of 1 mole carbon
= molar mass of c
= 12g
1mole of carbon is equal to 6.022 X 1023 atoms of carbon = 12g
Mass of 1 atom of carbon = 12/6.022 X 1023 that is nearly equal to 2 X 10 -23g
If the complete decomposition of Calcium Carbonate produces 1.4g of Calcium Oxide, what is the amount of Calcium Carbonate used and the amount of Carbon Dioxide produced?
CaCO3 (Calcium carbonate) → CaO (calcium oxide) + CO2 Carbon dioxide
Therefore X→ 1.4g + Y
The molar mass of Calcium oxide = 56g
Number of moles of CaO= 1.4/56, which is equal to 0.025
Molecular Mass of CaCo3 (Calcium carbonate) = 100g
Molecular Mass of CO2 (Carbon dioxide) = 44g
Here, 0.025 moles of CaCo3 = 0.025 X 100g
Which is equal to 2.25 of Calcium carbonate
And 0.025 moles of carbon dioxide = 0.025 X 44gg
Which is equal to 1.1f of carbon dioxide
Conclusion
A mole is a common scientific unit for measuring significant quantities of very small entities such as atoms, molecules, or other designated particles in chemistry.Mole is SI unit and denoted by mol. A mole is a measurement unit for the number of fundamental entities (atoms, molecules, and ions) present in a material. The term “mole” comes from a Latin word that means “a pile”. One mole is now regarded to be equal to Avogadro’s constant value. 6.022 x1023 entities are included in one mole. Number of Moles is given as the number of substances/number of moles. We can find the number of moles of atoms, gases, Particles too.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






