A solution is a homogenous mixture of 2 or more components. Each component of a homogenous solution has the same chemical composition. Therefore, the number of grams equivalent of an element dissolved in one litre of solution is the solution’s normality.
Define Normality and Its Formula
The term “normality” refers to the number of gram equivalents present in a litre solution. It is utilised in the preparation of acid or basic solutions. The units of concentration are
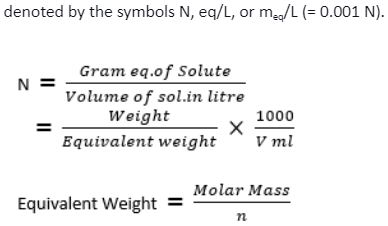
Where “n” denotes the number of H+ ions in an acid, OH- ions in a base, and charge available in ionic forms in a salt.
For example, 0.1 N HCl represents the concentration of hydrochloric acid. It is a unit of measure for the reactive capacity (or weight equivalent) of a certain chemical species expressed in grams (ion, molecule, etc.). The equivalent value is calculated using the chemical species’ molecular weight and valence. Normality is the primary concentration unit that the reaction activity can influence.
When the normality of a solution is 1, it is referred to as a normal solution. On the other hand, semi-normal and decinormal solutions have normalities of 1/2 and 1/10, respectively. These are referred to as the N, N/2, and N/10 solutions.
When Should You Make Use of Normality and Its Formula?
There are some instances why normality and its formula are preferred to molarity or another concentration unit for a chemical solution.
In acid-base chemistry, normality is used to define the hydronium (H3O+) concentration and hydroxide (OH-) concentration. In this case, the fraction 1/feq is a positive integer.
In precipitation reactions, the equivalency factor, also known as normality, is used to determine the number of ions likely to precipitate. Once again, 1/feq is an integer value.
In redox reactions, the equivalency factor specifies how many electrons an oxidising or reducing substance may donate or accept. 1/feq may represent a fraction in redox reactions.
Procedures for Solving Normality Issues
When calculating equivalents or reactant weights, it is essential to gather how many equivalents have been formed. Typically, you’ll need to consider the valence, molecular mass, and whether a material dissociates or dissolves completely.
Determine the solute’s gram equivalent.
Keep in mind that the volume of solution is measured in litres.
Using the normality and its formula, determine the normality.
Determine the solute’s equivalent weight in terms of the chemical reaction in which it will be used.
Formulas for Normality
There are several formulas for calculating normality. The appropriate one you choose will depend on the circumstances:
N = M x n
M is the molecular weight of moles per litre, and n is some equivalents formed. The number of equivalents is an integer for acid-base reactions, but maybe a fraction in a redox reaction.
N is equal to the number of gram equivalents divided by the litres volume of the solution.
=> N = Number of gram equivalents / volume of solution in litres
N = solute’s mass in grams / [solute’s volume in litres x equivalent weight]
N = Molarity x Acidity
N = Molarity x Basicity
N1 x V1 = N2 x V2
In a titration:
N1 = The acidic solution’s normality
V1 = Acidic solution’s volume
N2 = The basic solution’s normality
V2 = Basic solution volume
To make solutions of different volumes, you can use the following equation:
Initial Normality (N1) × Initial Volume (V1) = Normality of the Final Solution (N2) × Final Volume (V2)
An Example: The Normality of a Salt Solution
Calculate the normality of a 250 mL solution containing 0.321 grams of sodium carbonate.
The molecular weight of sodium carbonate can be calculated, and the ions it dissolves into can be identified using the formula. For example, sodium carbonate is represented by the formula Na2CO3 and has a molecular weight of 105.99 g/mol.
The sodium ions (2) and carbonate ions (1) are formed when it dissolves. Construct the problem in such a way that the units cancelled out to get an equivalent value per litre:
N = (mass in grams x equivalents) / (volume in litres x molecular weight)
Unit cancellation has been rewritten to make it more visible:
N = (0.250 L x (0.321 g) x (1 mol/105.99 g) x (2 eq/1 mol)
N = 0.0755 eq/L = 0.0755 N
The Limitations of Using Normality and Its Formula
There are a few things to consider when working with normality and its formula:
Normality demands an equivalence factor at all times.
Normality is temperature-dependent. It is stable as long as all experimental work is performed in the same environment (i.e., room temperature), but all bets are off once a solution is boiled or refrigerated. If you expect significant temperature variations, consider using a different unit, such as molarity or mass percentage.
Normality varies according to the material and chemical reaction in consideration. For example, if you compute an acid’s normality concerning a particular base, the result may differ if the base is changed.
Conclusion
Normality and its Formula isn’t the most common way to measure concentration, and its use is not acceptable for all chemical solutions under all circumstances. Normality is commonly used in acid-base chemical reactions, redox reactions, and precipitation reactions. It depends on the chemical process under observation and the temperature. So it refers to the chemical reaction. Equivalent concentration refers to the solution’s reactive capacity. The use of this is common in acid-base reactions and redox reactions.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




