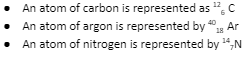We can measure molecular weight as a sum of the atomic weight value of atoms in a molecule. It can be used in chemistry to find stoichiometry in equations and chemical reactions and is abbreviated by M.W. or MW. The atomic weight of a molecule is measured in atomic mass units (amu) or Daltons (Da). The mass of isotope carbon-12, which is assigned a value of 12 amu, is used to determine both atomic and molecular weight. The atomic weight of carbon is not an exact 12 because it is a mixture of isotopes of carbon. The sample mass of a chemical compound, divided by the amount of material in that sample (determined in moles), is the molar mass of that compound.
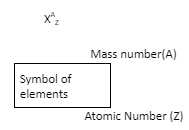
Mass Number
- The atoms of an element contain electrons, protons, and neutrons.
- Mass number of an element is the sum of the number of electrons, protons, and neutrons present in the atom of the element i.e.
Mass number of an element = number of protons + number of neutrons.
- Since protons and neutrons are present in the nucleus, these particles are collectively called nucleons. The mass number of an element is equal to the number of nucleons present in the atom of that element.
- The atomic number of an element is usually denoted by ‘ Z’ whereas its mass number is represented along with the symbol of the element.
Examples
Calculating a Mass Number
The mass number of an element may be calculated using the number of neutrons and the atomic number, or the number of protons or electrons in an atom.
Mass number = Atomic number + Number of neutrons.
If the other two numbers are known, the above method can be used to get any of the three values.
Example
Take the example of chromium. The atomic number of chromium is 24 and its mass number is 52. So how do we find out the number of neutrons present in the nucleus of an atom of chromium?
Solution
Mass number = Atomic number + Number of neutrons
Applying the value of atomic and mass number of chromium in the above formula we get:
Number of neutrons = mass number – atomic number
= ( 52 – 24)
= 28
Hence, there are 28 neutrons in the nucleus of chromium.
Molecular Weight
The molecular weight of an atom is calculated using its molecular formula. The atomic number of each atom is multiplied by its atomic weight, before being added to the weights of others. For example, the chemical formula of hexane is C6H14. Here, each hexane molecule has 6 carbon atoms and 14 hydrogen atoms. The atomic weights of Carbon and Hydrogen may be found on a periodic table.
To calculate the molecular weight of hexane, follow the steps below:
Atomic weight of carbon = 12.01
Atomic weight of hydrogen = 1.01
Molecular weight = (number of carbon atoms)(C atomic weight) + (number of H atoms)(H atomic weight)
Molecular weight = (6 x 12.01) + (14 x 1.01)
Molecular weight of hexane = 72.06 + 14.14
= 86.20 amu
Molar Mass
The mass of a mole of a given material is referred to as molar mass. The molar mass of a material is calculated by dividing its mass by its quantity. Kg/mol is the SI unit for molar mass. However, it is usually expressed as g/mol.
Molar mass = Mass of substance (Kg) / amount of substance (mol)
The atomic mass of an element is equal to its molar mass but units are different. As a result, a substance’s molar mass may be computed by adding the atomic masses of each element in the molecule.
Conclusion
In this article, we have tried to understand molecular weight and mass number along with the steps needed to calculate the same. The major distinction between molar mass and molecular weight is that molar mass refers to the mass of a mole of a certain material, whereas molecular weight refers to the mass of a specific substance’s one molecule. Molar mass and molecular weight have distinct definitions and units, but the value is the same. They are chemical analysis procedures that utilise the physical features of substances.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out