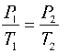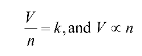According to Gay-Law, Lussac’s the pressure of a specified quantity of gas changes directly with the absolute temperature of the gas at constant volume. The main difference between Gay-Law Lussac’s and Charles’s Law is that the container in a Charles’s Law experiment is flexible, whereas the container in a Gay-Law Lussac’s experiment is rigid.
What is Gay Lussac’s Law?
The pressure of a gas sample in a solid container rises as the temperature of the gas sample rises. The molecules of gas impact the container’s surfaces with more force as their velocity rises, resulting in increasing pressure. The link between a gas’s pressure and its absolute temperature was found by French scientist Joseph Gay-Lussac (1778-1850).
Under a constant volume, Gay-Law Lussac asserts that pressure is exactly proportional to temperature.
According to Gay-Law, Lussac’s pressure is DIRECTLY proportional to the temperature, which means that when pressure rises, so does temperature.
Gay-Lussac’s Law:
Gay-Lussac’s Law also states that P ⁄ T = k, where k is the proportionality constant.
Where,
- P is the pressure
- T is the temperature
- K is the constant
A straight link may also be seen in a graph of pressure vs. temperature. When a temperature is reduced at a fixed volume, the pressure of the gas gradually lowers until it condenses into a liquid.

The starting pressure and temperature relationship for a gas with a given mass held at a constant volume are equal to the end pressure and temperature ratio, according to Gay-Law. Lussac’s
Mathematical explanation:
It can be expressed as follows –
P ∝ T
(when V= constant)
On removing proportionality –
p=kT …………. (1)
The ideal gas equation is given as:
PV=nRT ………….(2)
KTV=nRT
K=nR ⁄ V
K∝1 /V………..(3)
From the above equation, when K decreases, volume increases.
Law of combining volumes:
As soon as the temperature and pressure of the interaction gases and their products remain constant, the law of combining volumes states that when gases react, they do so in the volume with a simple whole-number ratio.
Simple whole numbers can be used to indicate the volume ratio between the reactant gases as well as the gaseous products.

For a reaction in which reactants and products are gases:
aA(g)+bB(g)→ cC(g)
The ratio of the volumes of gasses of A, B and C is a:b:c
Example:
- • When a pressurised aerosol could is heated, the increased pressure imposed by the gases upon that container (according to Gay-law) Lussac’s might cause an explosion. This is why many pressurised containers have warning warnings saying that they must be maintained in a cold environment and kept away from fire.
- • Thermostat: A pressure cooker is similar to an electric water heater. Steam is prevented from collecting by a pressure-relief valve. Heat causes the steam pressure within the heater to rise, eventually bursting it if the valve fails.
Limitation of Gay Lussac’s Law:
Only ideal gases are covered under the legislation. For actual gases at high temperatures and/or low pressure, Gay-law Lussac’s holds true. At high pressures, the pressure-to-temperature ratio shifted.
What is Boyle’s law?
- The inverse link between pressure and volume occurs according to Boyle’s Law
- but only if the number of molecules and the temperature are both constant.
- Boyle’s Law is used to forecast the outcome of changing the volume and pressure of a constant quantity of gas only, and only to the starting condition.
Where,
- P1 and V1 are the initial pressure and volume values and
- P2 and V2 are the values of the pressure and volume of the gas after the change.
What is Charles’s law?
Charles’ Law states that while pressure is maintained constant, the proportion of a given amount of gas changes dramatically with the absolute temperature of the gas. The absolute temperature is measured using the Kelvin scale.
Because zero on the Kelvin scale equates to a total suspension of molecular motion, it must be employed.
The direct relationship of Charles’s Law may be expressed mathematically by the following equation:
V / T=K
Where,
- V is the volume
- T is the temperature
- K is constant
What is Avagadro’s law?
Avogadro’s law states that equal volumes of various gases contain an identical number of water molecules under the same circumstances of temperature and pressure. The kinetic theory of gases may be used to determine this empirical relationship under the premise of a perfect (ideal) gas. For actual gases with sufficiently low high pressure and high temperatures, the rule is approximately correct.
Avogadro’s Law Formula:
At constant pressure and temperature, it can be expressed as:
V∝ n
V / n =K
Here,
- V is the volume of gas
- n is the amount of gaseous substance in moles
- K is a constant
Conclusion
As the temperature rises, the pressure of a gas rises as well. The Gay-Lussac Law outlines the relationship between pressure, temperature, and volume in a gas. The goal of this experiment is to understand better how pressure, temperature, and volume are related in a gas. In the first experiment, a syringe attached to a pressure sensor was used to compare the volume and pressure of a gas.
When pressure is held constant, Charles’ Law asserts that the proportion of a given amount of gas varies significantly with the actual temperature of the gas.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out