The lightest element, hydrogen, exists in its standard elemental form as a diatomic molecule, called dihydrogen, H2. The atomic structure of hydrogen can only exist at high temperatures. The diatomic form of hydrogen consists of two hydrogen atoms. Each atom comprises a proton in its nucleus and an electron around it. Just like the electron, the proton is also spinning about an axis.
The two protons of the hydrogen molecule may be either spinning in the same direction or opposite directions in their nucleus, giving rise to two forms of hydrogen, i.e., ortho and para.
Ortho hydrogen
Ortho hydrogen molecules are in which the spin of both protons in the nucleus is in the same direction. The Ortho form of hydrogen is more stable than the para form, but it is challenging to obtain a pure ortho form.
Physical properties of ortho hydrogen:-
- Ortho Hydrogen Shows parallel nuclear spin
- The Nuclear spin of ortho hydrogen = ½ + ½ = 1
- The Ortho form of hydrogen is stable at room temperature.
- The melting point of ortho hydrogen is 13.95 K.
- The boiling point of ortho hydrogen is 20.39 K.
- The magnetic moment of ortho hydrogen is twice that of a proton.
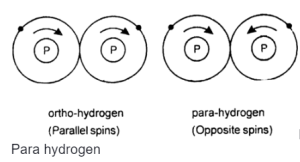
Para hydrogen molecules are defined as the ones in which the spins of both the protons in the nucleus are in opposite directions.
Physical properties of para hydrogen:-
- Para hydrogen shows antiparallel spin
- The nuclear spin of para hydrogen = ½ – ½ = 0
- Para form of hydrogen is more stable at a lower temperature.
- The melting point of para-hydrogen is 13.83 K.
- The boiling point of para-hydrogen is 20.39 K.
- The magnetic moment of para-hydrogen is zero as the spins neutralise each other.
- The vapour pressure of liquid para-hydrogen is higher than that of liquid ortho hydrogen.
- The internal molecular energy of para-hydrogen is lower than the ortho form of hydrogen.
Preparation of para hydrogen
Para hydrogen was initially prepared by absorbing ordinary hydrogen in activated charcoal in a quartz vessel. The solution was maintained at a temperature of 20K. The charcoal absorbs almost pure para hydrogen. Hence, by this method, pure para-hydrogen can be isolated.
Hydrogen in its ordinary form exists as an equilibrium mixture of ortho and para hydrogen.
Ortho hydrogen ⇌ Para hydrogen
The following methods carry transformation of para form into ortho form of hydrogen.
- When treated with catalysts like platinum or iron.
- When an electric discharge is passed.
- When heated at 800°C or higher.
- When mixed with paramagnetic molecules like O2, NO, NO2.
- When mixed with nascent Hydrogen or atomic Hydrogen.
Ordinary hydrogen at room temperature and normal atmospheric conditions is a mixture of ortho-form and para-form in the ratio of 3:1, i.e., ordinary hydrogen consists of about 75% ortho and 25% para form.
Dependence of ortho and para form of hydrogen in ordinary hydrogen on temperature
As the temperature decreases, the equilibrium shifts in favour of para hydrogen.
- At 0°K, hydrogen comprises mainly the para form of hydrogen, which is more stable.
- At the temperature of air liquefaction, ordinary hydrogen comprises 50% ortho and 50% para hydrogen.
- Ordinary hydrogen comprises about 75% ortho and 25% para form at standard temperature and atmospheric conditions.
- At shallow temperature, say about 20K, 99.82% para, and only 0.18% ortho is present.
- As the temperature rises, the percentage of ortho form of hydrogen in ordinary hydrogen increases while the para form decreases, reaching the limiting ratio of 3:1.
- Even at significantly high temperatures, the ratio of ortho to para-hydrogen can never be more than 3: 1.
This conversion of ortho form into the para form on increasing temperature shows that the intrinsic energy of ortho form is higher than para form.
Though the two forms differ in physical properties, they show similar chemical properties.
Conclusion
Depending upon the direction of spins of the protons in nuclei of diatomic hydrogen, hydrogen is of ortho and para form, which exist as an equilibrium mixture at room temperature in a ratio of 3:1. Among the two states of hydrogen, though the ortho form of hydrogen is more stable than the para form, it is challenging to obtain a pure ortho form. The two forms differ in physical properties, but they show similar chemical properties.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






