Its equivalent mass is the number of parts by mass of a material that combines with or displaces 1.008 parts by mass of hydrogen, 8.0 parts by mass of oxygen, or 35.5 parts by mass of chlorine (EM).
The gramme equivalent mass (GEM) or gramme equivalent is the quantity of material in grammes numerically equal to its equivalent mass.
There are various ways to calculate the equivalent mass of metal.
The equivalent mass is used in general chemistry, volumetric analysis, gravimetric analysis and polymer chemistry.
As we know, The elements are always present in exact amounts by mass when atoms combine to create chemical compounds. This fact may be utilised to make chemically distinct molecules with different masses equal.
Let’s consider the following compounds
HCL: as we know, the compound is made up of Hydrogen and Chlorine, where 1g of Hydrogen reacts with 35.5g of Chlorine
AgCl : Here, Agcl is the compound consisting of silver and Chlorine, where 108g of silver reacts with 35.5g of Chlorine.
From the above compounds, we can deduce that 1g of H2 ≡ 108g of Ag.
According to the Laws of chemical combination, all elements combine. The value of the equivalent mass of an element is the number of parts it mixes with 1 part by mass of Hydrogen, eight parts by mass of oxygen, 35.5 parts by mass of Chlorine, or one gram equivalent of any other element.
The valency of an element determines its equivalent mass. The formula for calculating an element’s equivalent mass is given as
Equivalent Mass = Valency/ Atomic Mass
There are several methods used.
Method of Displacement of Hydrogen
We already know that metals react with acids to form hydrogen, and the hydrogen displacement technique simply uses this fact to determine the equivalent mass. Under average temperature and pressure, some mass of an element interacts with an acid to produce some quantity of hydrogen gas (STP).
How can we equate such elements?
It’s like balancing two chemically distinct molecules, which is equivalent to combining two bananas and three apples!
The mass of metal necessary to displace 11200cm3 of hydrogen, as we learnt in the mole idea, is nothing more than 1g of hydrogen. As a result, we arrive at the following formula:
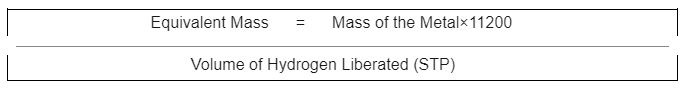
3g of metal reacts with dilute HCl and liberates 2800 cm3 of hydrogen gas at STP.
Calculate the equivalent mass of the metal
Solution: Given values As per the previous equation
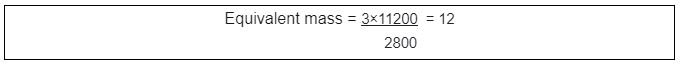
The Oxide Method
We discovered that metals react with oxygen to form metal oxides. The oxide technique calculates the mass of metal oxide formed when a given amount of metal easily interacts with oxygen. Then, because we require a standard ratio, we calculate the mass of the metal that combines with 8g of oxygen, which is the metal’s equivalent mass:
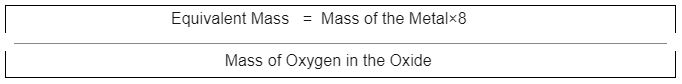
The mass of metal In its oxide is 75% of the mass of the whole compound. Calculate its equivalent mass metal.
Solution: Let us assume that the mass of the metal oxide is X. then the mass of the metal would be 75 x, and the mass of the oxygen in it would be 100
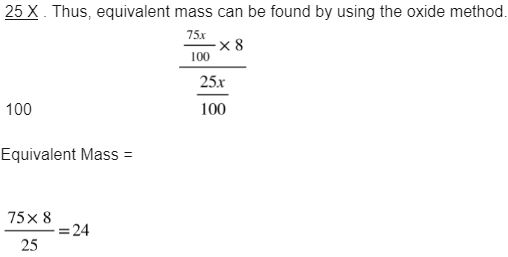
Chloride Method
This is an additional way for calculating the equivalent mass when the first two approaches are incompatible. When a mass of an element reacts with chlorine to form the corresponding chloride, we compute the ratio at which a metal mixes with 35.5text g35.5 g of chlorine, giving us the equivalent mass.
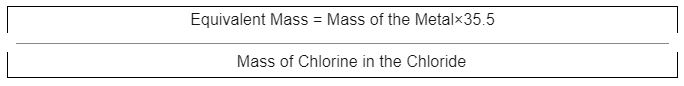
Fifty-four grams of silver reacts with HNO3 to form silver nitrate; later displacement reaction occurs when NaCl is added to AgNo3 to form 7.75 G of all. Find the equivalent mass of the silver.
Solution: The mass of agcl formed is 17.75 5G, and the amount of chlorine inside it is 17.75 – 54 equals 17. 75 grams; thus, plugging these values in the chloride equation, we get
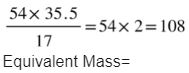
Equivalent mass masses can be found in compounds too.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



