Metals and their sulfides will not mix with the dispersion media to generate a friction solution. Their colloidal solution is produced using some unique ways. Lyophobic soles are the name for these types of soles. There is always some charge on these types of colloidal solutions. The stability of the colloidal sols is determined by their charge.
Colloidal solutions have a charge that reflects their stability. If we could remove the charge from the fluid, the cells would become closer to one another and aggregate, producing sediment and gravel under gravity’s action. Precipitation or coagulation is the accumulation and settlement of the cell process. Because the coagulation process can be done in a variety of ways, the various coagulation procedures are briefly described here.
By electrophoresis:
This forces the colloidal particles to travel towards the anti-charged cells, after which they are released and collected at the bottom.
By mixing of oppositely charged particles:
The anti-charged particles are combined in equal numbers and precipitated by dissolving their charges in this type of coagulation process.
By boiling:
When we boil a solution, the diffusion medium molecules begin to collide with each other and with the surface, disrupting the absorption layer. The charge on the solution is reduced, causing the cells to settle.
Persistent dialysis:
The electrolytes are totally removed by continuous dialysis components, and the solution loses its stability and eventually freezes.
Hardy-Schulze’s rule:
The higher the valence of the anti-charged ion of the electrolyte is added, the faster the clotting, according to Hardy Schulz’s law. The electrostatic attraction force principle. The coagulation force is proportional to the valence of the flocculation ion.
According to this rule,
- The ions that have the opposite charge to the sol cells cause the sol to coagulate.
- 2Coagulating power of an electrolyte is directly proportional to the fourth power of the valency of the active ions (ions causing coagulation). Greater is the valency of the oppositely charged ion of the electrolyte being added, the faster is the coagulation.
- The fourth power of the active ions is directly related to the freezing point of the electrolyte (causing the ions to coagulate). The higher the valence of the opposing charged ion in the electrolyte, the faster it coagulates.
- To coagulate the positive sol in the same way. The freezing points of various ions, such as , were discovered to decrease in order., were discovered to decrease in order.
- The coagulating value or flocculation value is the lowest concentration of electrolyte that causes the sol to coagulate.
Some Coagulating values of Cations and anions are as follows;
Electrolyte | Cation and anion | Coagulating value |
| NACl | NA+ | 52 |
| MgCl2 | Mg2+ | 0.72 |
| BaCl2 | Ba2+ | 0.69 |
| AlCl3 | Al3+ | 0.093 |
| K br | Br- | 138 |
| K2SO4 | So2 4- | 0.210 |
| K2C2O4 | C202 4- | 0.238 |
| K2C2O4 | C202 4- | 0.238 |
![]() Coagulation of lyophilic solutions:
Coagulation of lyophilic solutions:
Lyophilic solutions are more stable due to the presence of charged particles and Solvation of the colloidal particles. The coagulation of lyophilic solutions is done by adding electrolytes or suitable solvents.
Protection of colloids:
The addition of modest amounts of electrolytes to lyophobic sols, such as metals like gold and silver, quickly precipitates them.
The stable lyophilic colloids like gelatin and albumin might have been added to keep them from coagulation. This is because when lyophilic sol is given to a lyophobic soul, the lyophilic particles build a protective membrane around the lyophobic particles.
If a small amount of gelatin is added to gold sol, it is not readily precipitated by the addition of sodium chloride. This process of protecting the lyophobic colloidal solutions from precipitation by the electrolytes due to the previous addition of some lyophilic colloid is called protection. The colloid which is added to prevent coagulation of the colloidal sol is called protecting colloid.
When a small amount of gelatin is added to the gold sol, it does not precipitate when sodium chloride is added. Protection is the process of introducing some lyophilic colloid to shield lyophobic colloidal solutions against precipitation by electrolytes. Protecting Colloid is a colloid that is introduced to prevent the colloid sol from coagulation.
Gold number:
The gold number is the number of milligrams of protective colloid required to avoid the coagulation of 10 ml of standard gold solution when a 10% solution of 1 ml sodium chloride is added. If the substance has a small value of gold number it protects more.
Substance | Gold number |
Starch | 20 -25 |
Gum arabic | 0.15 – 0.25 |
Egg albumin | 0.1 -0.2 |
Hemoglobin | 0.03 -0.07 |
Gelatin | 0.005-0.01 |
Conclusion:
The process of colloidal particles aggregating and settling as sediment is known as coagulation. When metals and their sulfides come into contact with the dispersion media, they do not form a colloidal solution. According to Hardy Schulz’s law, the higher the valence of the anti-charged ion of the electrolyte added, the faster the clotting. The principle of electrostatic attraction. The coagulation force is proportional to the flocculation ion’s valence.
The gold number is the amount of protective colloid required to prevent 10 ml of standard gold solution from coagulating when a 10% solution of 1 ml sodium chloride is added. It protects more if the substance has a low gold number value.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out





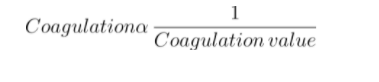 Coagulation of lyophilic solutions:
Coagulation of lyophilic solutions:
