Chemistry is often said to be the study of the composition of matter and the chemical changes it undergoes. What does matter mean, to begin with? It is anything that has a mass and takes up a certain amount of space. Everything is made of matter, from the air you breathe to anything you drink. The elementary particles with which matter is made are called atoms. Several atoms join together to form molecules. Different kinds of matter are categorised based on multiple factors, including physical and chemical properties.
There are multiple classifications of matter based on their composition and physical state. All of these types have distinctive properties and chemical compositions. Let’s now look at the parameters that act as a basis for the classification of matter.
Physical and chemical characteristics of matter
Classification of matter in chemistry categorises all mixtures and substances according to their different chemical and physical properties. Physical characteristics include size, colour, shape, temperature, boiling points, melting points, density, and mass. These properties define the physical nature of matter.
On the other hand, chemical characteristics define the chemical elements present in the compound. Chemical properties also describe the changes in its identity to form new substances.
For example, water boils at 100 degrees Celsius and freezes at zero degrees Celsius. These define its physical properties. Two atoms of hydrogen and one atom of oxygen together produce the compound water. Other chemical properties include pH levels and the amount of oxygen dissolved in the solution. There is approximately 88.9% of dissolved oxygen in water. Similarly, on being subjected to electrolysis, the compound chemically changes to hydrogen and oxygen elements.
Classification of matter based on the chemical composition
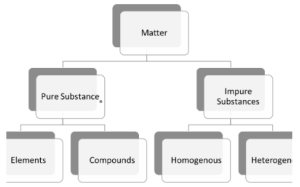
Broad classification of matter in chemistry includes dividing them into:
- Pure substances: These substances are made of similar atoms or molecules in a defined ratio. Therefore, they have a homogeneous chemical composition. Pure substances have a fixed structure and definite melting and boiling points.
This classification of matter, i.e. the pure substances, can be further classified into two types.
- Elements
Elements are defined as pure substances that contain a single type of atom or molecule. They can’t be broken down further by any physical or chemical means. At present, there are about 118 elements, out of which only a few are commonly found around us. Examples of elements are silver (Ag), gold (Au), sulphur (S), hydrogen (H), etc.
- Compounds
A compound is formed when more than one element combines chemically in a fixed ratio. Pure compounds can be chemically disintegrated or transformed into newer substances. Examples of these substances are salt (NaCl), carbon dioxide (CO₂), water (H₂O), etc.
- Impure substances: They are made of different types of atoms or molecules. Unlike pure substances, their structure isn’t fixed or constant. Impure substances can be segregated into pure forms with the help of purification methods like sublimation, filtration, crystallisation, etc.
This classification of matter can be further subdivided into two categories based on their composition:
- Homogeneous mixture:
Homogeneous mixtures have the same composition throughout their volume. You can’t see the individual particles in a homogeneous mixture. The amount of substances present in every sample of a homogeneous mixture is always the same. Examples of homogeneous mixtures are oil, wine, saline water, etc.
- Heterogeneous mixture
A heterogeneous mixture, on the contrary, has varied compositions throughout its volume. Unlike homogeneous mixtures, these mixtures don’t blend perfectly. Therefore, you can determine what substances these mixtures are composed of. Examples of heterogeneous mixtures include mud water, concrete, chocolate chip cookies, etc.
Classification of matter based on the physical state
The classification of matter based on their physical states involves the following states:
- Solid-state: The solid-state of matter has a definite shape and volume due to its closely packed particles. The atomic bond between the particles in a solid is so strong that it resists their free movement. However, solids can undergo vibration. They may break if you press a solid, but their shape can’t be altered. That is why solids are the most inflexible of all forms of matter. Examples are rocks, ice, wood, etc.
- Liquid state: Liquid particles have more space among them than solid particles. That is why these particles are capable of free movement. Liquids, unlike solids, don’t have a definite shape, but they do have a definite volume. They can alter their shape and take the volume of the container in which they are kept. Examples are water, oil, petrol, etc.
- Gaseous state: This phase of the matter doesn’t have any fixed shape or volume. Gases are highly compressible, and their diffusion rate is quite higher than both solids and liquids. The gas molecules have a huge amount of space between them. They are capable of free movement and can move around at random. Examples: carbon dioxide, air, etc.
For better understanding and help in revision, the classification of matter chart is given below:
Conclusion
The matter is regarded to be anything that has volume and mass. The classification of the matter is undertaken based on its physical state or chemical composition. The matter is classified as impure and pure substances or into the solid, liquid, or gaseous states. Chemical properties like reactivity, flammability, pH levels, chemical stability, and oxidation states also play a role in the classification of matter. Pure substances are further categorised as elements or compounds. Impure substances are categorised into homogeneous or heterogeneous mixtures. The classification of matter charts in the study notes shows this categorisation in an organised way.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out