In the earlier times, Greeks considered four elementary substances as the Universes’ basic component. Air was known as one of these four substances, and the other three substances included fire, water and earth. However, as we move forward in time, around the year 1800s, John Dalton, a well-known scientist, identified that many different chemical gases composed the atmosphere. As we move forward, we will be focusing on the composition of the atmosphere in a detailed manner.
Composition of Atmosphere
John Dalton had recognized some crucial elements which formed the atmosphere. These elements included oxygen, nitrogen and another element that was present in small proportion and was not combustible, later it was identified to be argon. Thus, this helped in learning a little about the composition of the atmosphere. In the 1920s as the spectrometer had developed scientists now researched those gases in the atmosphere which were present in very small proportions. These gases include carbon dioxide and ozone. Even though the proportion of these gases were less yet the proportion had variations based on different places. The table below helps to understand some proportions of gases that remain constant in proportion.
Name of The Gas | Proportion in percentage |
Nitrogen | 78.08% |
Oxygen | 20.95% |
Argon | 0.93% |
Helium, Neon and Krypton | 0.0001% |
Table 1
In another table below, we have mentioned some of those gases whose proportions are not constant and can vary in different times and places.
Name of the Gas | Proportion |
Carbon dioxide | 0.038% |
Water vapour | 0 to 4% |
Methane | Trace |
Sulphur dioxide | Trace |
Ozone | Trace |
Nitrogen oxides | Trace |
Table 2
Thus, the tables help in giving a comprehensive view of the composition of the atmosphere. Apart from these significant gases the atmosphere also has various layers.
Thus, as observed from the tables above, Nitrogen is the gas that covers the majority of the atmosphere. The next to Nitrogen is Oxygen which is also found in significant amounts. Lastly, elements such as carbon dioxide, argon and other gases constitute the atmosphere in comparatively lesser proportions.
Layers of Atmosphere
The various layers of the atmosphere include the:
- Ionosphere,
- Mesosphere,
- Stratosphere
- Troposphere.
In the ionosphere, there is very high average kinetic energy for the particles. Further, in this layer, the density of the gas is less. Matter exists in the form of atoms in the mesosphere. The Stratosphere is the layer that can be called the ozone layer’s home. As the altitude increases, the temperature rise can also be observed in this layer. Lastly, the troposphere is the one that can be called nearest to the Earth. It is the area where all weather-related events occur. Thus, these are some significant layers of atmosphere and each one has its own notable features.
Conclusion
As observed from above, various elements constitute the atmosphere. Each element has its own features, proportion and role to play in the atmosphere. Nitrogen is the one which can be found in the highest proportion that is about 78% and then comes Oxygen which is for about 21%. There are several other gases as well present in comparatively lesser proportions such as argon, helium, carbon dioxide etcetera. We also studied the various layers of the atmosphere and learnt about the ionosphere, mesosphere, stratosphere and troposphere. Thus, it can be concluded that the various components of the atmosphere, as well as the layers of the atmosphere, have been clearly understood. Overall, the composition of the atmosphere is now clear and well understood.
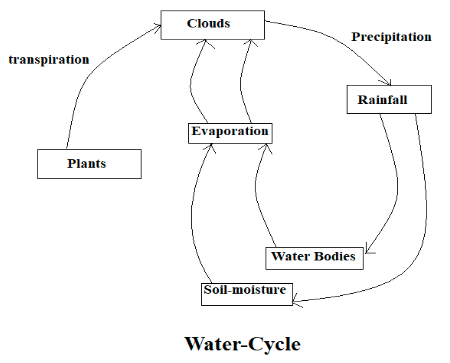
Water Cycle
Due to the heat of the sun water evaporates from lakes, seas, rivers, and other water bodies; this process is known as evaporation, water also evaporates from the plant’s leaves, which is known as transpiration. As the water rises from lakes, seas, rivers, and other water bodies in the atmosphere, it gets cool and condenses to form clouds as a continuous process of evaporation and condensation, gradually the clouds get heavy, and as a result, precipitation occurs in the form of rain. And this rainwater is stored in any of the following reservoirs such as the ocean, soils, glaciers, under the Earth’s surface as groundwater, lakes, snowfields, etc.
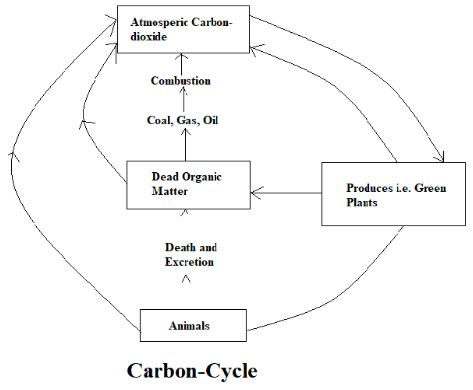
Carbon-Cycle
The carbon concentration in the atmosphere is about 0.03%. Circulation of carbon from the atmosphere to the biosphere, hydrosphere, lithosphere, and returns to the atmosphere is known as the carbon cycle. The carbon from the atmosphere enters into the plants through photosynthesis for synthesizing food particles, when the plants are consumed by terrestrial organisms, carbon dioxide enters them. When these terrestrial organisms die the carbon dioxide enters the soil. Carbon dioxide enters into the water as carbonates, which helps the aquatic plants to perform photosynthesis. CO2 returns to the atmosphere from fossil fuel, from respiration of animals, the release of smoke from vehicles, from the burning of wood. If CO2 increases in the atmosphere it disturbs the natural carbon cycle.
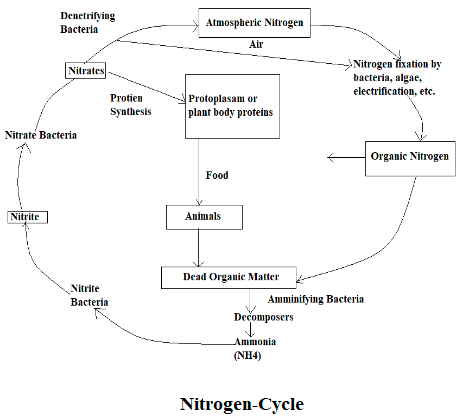
Nitrogen Cycle
The atmosphere is made up of 78% Nitrogen; we need nitrogen for DNA and proteins. Nitrogen that is available in the atmosphere cannot be directly consumed by plants, they can consume the nitrogen when it is fixed i.e. when combined with other elements like carbon, hydrogen, and oxygen. By the action of denitrifying bacteria, nitrogen is spontaneously entering into the air and through the action of electrification and lightning, nitrogen is entering into the soil.
Conclusion
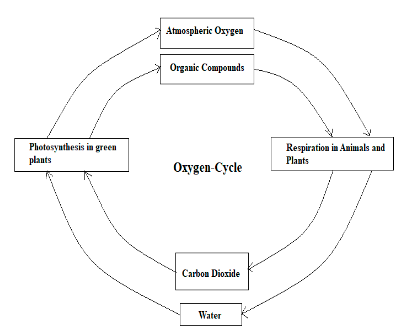
It is to conclude that the biochemical cycle in an ecosystem is defined as the transformation and transport of chemicals in ecosystems. In wetlands, by unique hydrological conditions, biogeochemical cycles are strongly influenced. There are four main important biogeochemical cycles in the ecosystem are oxygen cycle, water cycle or hydrologic, carbon cycle, and nitrogen cycle
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




