Thermal energy can be referred to as the energy which a system or substance has because of its temperature. The heat makes the molecules of that substance or system move or vibrate. Due to thermodynamics, the atoms or molecules of a substance or a system move and interact with each other in an extremely complicated manner but even after that, they can reach a state of equilibrium if they match certain criteria. These interactions can be idealized as the pressure of the system, mass of the system, volume, or any other. It is important to state that the energy which is acquired, comes from the transfer of heat energy between two masses of matter due to differences in the temperature between them. The energy which is generated in the whole process keeps on losing a part of itself due to entropy. As we all know that heat, being a form of energy, cannot be created or it can be destroyed and can only be transferred. In thermodynamics, the same law applies too. There are other three laws of thermodynamics too which are based on the zeroth law of thermodynamics which again, acts as the fundamentals for the corresponding laws. The zeroth law of thermodynamics is concerned with thermal equilibrium and transfer of heat energy.
Zeroth Law of Thermodynamics
The Zeroth Law of Thermodynamics- According to the Zeroth law of thermodynamics, if two thermodynamic systems are in thermal equilibrium with a third one, then they are in thermal equilibrium with each other. The law simply follows the concept of temperature.
Therefore, in mathematical expression, the law can be stated as:
If a=c and b=c, then a=b. (Here, a, b, and c represents three different thermal bodies where a and b have the same temperature and the temperature of c is both equal to a and b. therefore, a=b=c).
Illustration of the law with the help of a diagram:
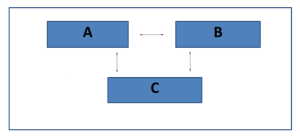
Figure 1: Illustration of the Zeroth Law of Thermodynamics
In the above diagram, each of the three blocks represents three different bodies having different temperatures at first but eventually, heat flows from the comparatively hotter object to the colder object until all the objects reach a state of equilibrium in terms of temperature. The flow of heat can be in any direction depending on the temperature.
Examples of Zeroth Law of Thermodynamics
Example 1: The example of a thermometer in a room is one of the best examples of the zeroth law of thermodynamics. Suppose that the thermometer reading says that the temperature is 28 degrees Celsius. Therefore, the temperatures of the thermostat and the room are the same and any random object in the room will also have the same temperature because objects in the room and the room itself have the same temperature.
Example 2: If you take a glass of hot water and a glass of cold water and put both the glasses in the refrigerator, after a considerable period, say four hours, both the contents of the water will have the same temperature as the temperature inside the refrigerator as the refrigerator has cooled both the glasses until they have reached their coldest possible temperature and attained thermal equilibrium.
Example 3: Consider that you put a packet of raw fish in the fridge which has other items inside it too. In the next morning, the temperatures of the raw fish, other items, and the fridge’s interior itself will all have the same temperature. This is another prime example of the zeroth law of thermodynamics.
Importance of the Zeroth Law of Thermodynamics
The zeroth law acts as the fundamentals of the other laws of thermodynamics and the overall core concept of thermodynamics in general. With the help of the zeroth law, the concept of temperature scale can be understood. The thermal equilibrium between two objects and their relationship with a third element if any one of the first two has the same temperature with the third can be proved by this law (If a=c and b=c, then a=b). Here, a, b, and c represents three different thermal bodies where a and b have the same temperature and the temperature of c is both equal to a and b.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




