In biological processes, enzymes act as catalysts. These enzymes carry out a number of functions that mediate the enormous variety of metabolic events that make up life. These macromolecules’ catalytic competence and selectivity stand out as their most notable qualities.
An enzyme selects which of numerous possible chemical reactions might actually occur in a biological system by attaching to a specific substrate and catalyzing it. The majority of metabolic events in biological systems do not occur at observable speeds in the absence of enzymes because they are accelerated by factors of up to a million or more by enzymes.
Some Characteristics of Enzyme
- Except for a tiny subsection of catalytically active RNA molecules, or ribozymes, all enzymes are proteins
- Enzymes have extremely strict substrate specificity
- In effect decreasing the activation energy, enzymes can speed up a biological reaction
- Enzymes alter the rate at which the equilibrium is reached, not the equilibrium state, of the biological reaction on which they are operating
- At the conclusion of the reaction, the enzyme is unaffected
- The initial portion of an enzyme’s name typically identifies the substrate on which the enzyme functions, and the majority of enzymes typically end with the suffix “-ase.” For instance, the enzyme amylase reacts with the substrate starch or amylose
Mechanism of Enzyme Action
- The substrate is initially recognized by the enzyme (E) and bound to its active site (S).
- The development of the activated enzyme-substrate complex (ES) is the next step,
- Lastly the enzyme works on the specific bonds in the substrate to change it and create the product (P).
The enzyme ultimately decreases the energy barrier during the entire process, enabling the organisms to carry out reactions more quickly.
In the mechanism of enzyme action, substrate-specificity of the enzyme is also crucial. There are now two different theories that describe how an enzyme works in this regard.
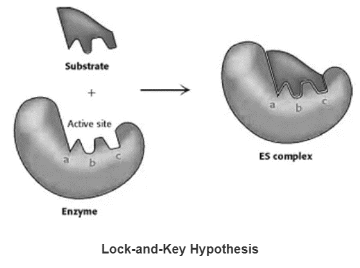
Lock- and-Key Hypothesis
The unbound enzyme’s active site has a complementary shape to the substrate.
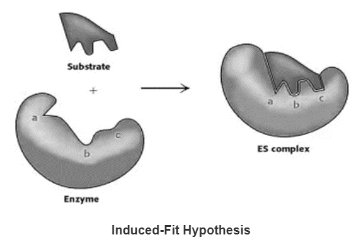
Induced-Fit Hypothesis
Only after the substrate has been bound does the flexible enzyme’s active site take on a shape that is complementary to the substrate.
Basic Structure and Cofactor of Enzymes
The three-dimensional cleft or crevice region where an enzyme binds its substrates is known as the active site. It also includes the leftovers referred to as catalytic groups.
Many enzymes require the presence of tiny molecules known as cofactors in order to function catalytically. A complete, catalytically active enzyme is referred to as a holoenzyme, while such an enzyme without its cofactor is known as an apoenzyme.
Small organic molecules and metal ions are the two main categories of cofactors.
For instance, the enzyme carbonic anhydrase needs Zn2+ to function, while the enzyme glycogen phosphorylase needs the tiny chemical compound pyridoxal phosphate to utilize glycogen for energy (PLP).
The small organic molecules, known as coenzymes, are primarily generated from vitamins and can either temporarily or permanently remain loosely linked to the enzyme; in this instance, they are referred to as prosthetic groups.
How to use Enzymes in a Catalysis?
In a biological system, every chemical reaction has an energy barrier that prevents it from happening spontaneously. The activation energy is the amount of energy needed to initiate a reaction and break through this energy barrier. Enzymes assist reactions to accelerate in accordance with the demands of the cell by lowering the activation energy.
Where substrate and enzyme are combined, a new reaction pathway is formed whose transition-state energy is lower than that of the process without the enzyme (the precise molecular point throughout the reaction when the reaction can progress either towards substrate or product).
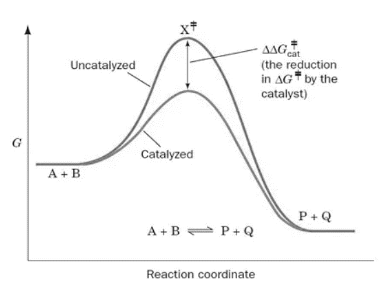
The progression of the reaction is shown in the above image both with and without an enzyme. The DG, or the free energy of activation, is decreasing, which accelerates the enzyme-catalyzed process. The substrates are A and B, the products are P and Q, and the transition state is X.
Factors Affecting Enzyme Catalysis
The pH, temperature, substrate concentration, and enzyme itself can all have a significant impact on an enzyme’s activity. These parameters all influence the structure of the enzyme, which is a protein.
pH and Temperature
Every enzyme has a specific pH and temperature range where it performs at its peak, known as the optimum pH and optimum temperature, respectively. Above and below these ranges, the activity of the specific enzyme decreases.
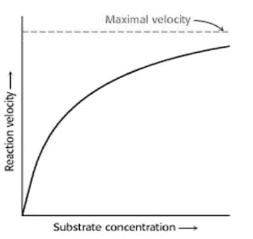
Substrate Concentration
The speed of an enzyme-catalyzed reaction is primarily influenced by the substrate concentration; initially, the speed rises with an increase in substrate concentration and then reaches a maximum level from which it can never again rise, whether or not the substrate concentration is still increasing.
Allosteric Regulation
In order to accept specific modulator or effector molecules that ultimately cause conformational changes in these allosteric enzymes and may potentially influence their catalytic activity, some enzymes contain an additional binding site known as an allosteric site or regulatory site. These modulators can either be inhibitors or activators, decreasing the activity of the enzyme.
Conclusion
Thus, it may be argued that the most important protein for metabolism in the human body is an enzyme. They are referred to as biological catalysts since they speed up chemical reactions in our bodies. An enzyme-substrate complex is created when an enzyme binds to its substrate.
The fact that enzymes are ultimately unaffected by the reactions they catalyze is one of their key characteristics. You will discover all of the enzymes’ tasks in this article, along with their illustrations, and numerous other details.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






