Biocatalysts called enzymes accelerate biological processes. In actuality, genes have encoded them as protein molecules. Enzymes come in a variety of varieties. Different alleles of the same gene are located at the same locus code for certain enzymes. Allozymes are the name for them. Some enzymes, however, are encoded by distinct genes that are found at several loci. Isozymes are the name for these enzymes. It is possible to establish the link between two species using isozymes and allozymes.
How Do Allozymes Work?
Distinct alleles present at the same locus can produce different enzymes known as allozymes. Allozymes are therefore encoded for by several alleles at a single locus. They exhibit a little change in their structure or amino acid sequence. They serve a very similar purpose, though. Allozymes differ somewhat from one another owing to random variations in DNA sequences known as mutations. Based on the molecule sizes and electrical charges, capillary electrophoresis may identify these minute changes.
It is possible to employ allozymes as a species-relatedness indicator. As a result, they serve as helpful markers for tracing family trees across several species within the same genus and can help explain how an organism evolved. In order to map closely related species in many groupings, including plants and animals, allozymes are widely utilised. There would be less variations between allozymes if the species were closely related.
How do Isozymes Work?
Isozymes are variations of an enzyme that are produced by various genes at various loci. Isozymes can be defined as distinct versions of an enzyme that are encoded by various genes. Isozymes’ structural differences from their amino acid sequences are marginal. As a result, they come in a variety of sizes and forms. However, they both catalyse the same biological process.
However, they are able to function in a number of circumstances, at various points in our bodies, at various phases, or in varied cellular situations.
Are Isozymes the Same as Enzymes?
Isoenzymes, also known as isozymes, are alternate manifestations of the same enzyme activity that occur in various ratios in various tissues. Isoenzymes vary in their multimeric quaternary structure, amino acid sequence, and content; often, but not always, they have comparable (conserved) structures. Their expression in a certain tissue depends on how the gene for each component is controlled. According to its function in that tissue, each isoenzyme form will have unique kinetic and/or regulatory characteristics. Typically, electrophoresis is used in clinical laboratories to identify isoenzymes.
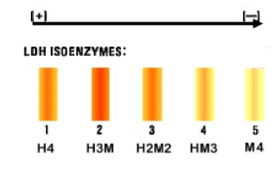
Because of the availability of isozymes, metabolism may be tailored to suit the unique requirements of a certain tissue or developmental stage (for instance, lactate dehydrogenase (LDH)). Isoforms, or closely similar variations of an enzyme, are referred to as isozymes (or isoenzymes) in biochemistry. They frequently have homologous genes that have diverged over time acting as their code. Although technically speaking, isozymes and allozymes refer to enzymes from various genes that process or catalyse the same reaction, and allozymes refer to enzymes from different alleles of the same gene, respectively, the two terms are frequently used interchangeably.
How Isozymes Regulate Enzyme Activity?
Isozymes, sometimes called isoenzymes, are structurally distinct homologous enzymes that catalyse the same process. The isozymes’ variations enable them to control the same response at several locations throughout the species. They differ specifically in the order of the amino acids. They exhibit diverse regulatory and kinetic characteristics. For instance, isozymes can be identified from one another by biochemical traits like electrophoretic mobility, which have differing KM and Vmax values.
Isozymes are expressed in certain organelles or at specific developmental stages and are encoded by several genes. Isozymes have the ability to fine-tune metabolism to suit the demands of various developmental stages and support the normal functioning of various tissues and organs in accordance with their physiological make-up and environment of function. For instance, the amino acid sequences and levels of expression of the isoenzymes of lactate dehydrogenase in various animal organs varies. The amount of oxygen delivery affects the concentration of various isozymes in a given organ. Isozymes are found in particular bodily parts and differ in particular organelles or tissues.
Differences of Isozymes and Enzymes
An enzyme known as an allozyme can be produced in several forms by a single gene. An enzyme known as an isozyme, on the other hand, is produced in many forms by various genes. This is the primary distinction between isozymes and allozymes. While distinct genes at separate loci code for isozymes, various alleles at the same locus code for allozymes.
| Enzymes | Isozymes |
|
|
|
|
|
|
|
|
Conclusion
Isozymes often develop through gene duplication, although they can also happen as a result of polyploidization or hybridization. If the new variant’s function stays the same as the original during the course of evolution, it is likely that one or the other will be lost as mutations build up, resulting in a pseudogene. The two versions may both be favoured by natural selection and become specialised to separate roles if the mutations do not instantly prevent the enzyme from functioning but instead affect its function or pattern of gene expression. They could express themselves in various tissues or at various developmental stages, for instance.Point mutations or insertion-deletion (indel) events that change the dna coding sequence of the gene can result in allozymes.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out