Cleavage of Bonds
The breaking and formation of bonds occurs in organic chemistry processes. Bonds can be severed in one of two ways:
- Homolytic Cleavage
- Heterolysis Cleavage
What is homolytic cleavage and how does it happen?
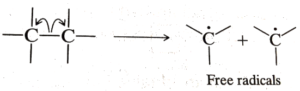
Homolytic cleavage occurs when the covalent bonds between two elements break in such a way that each element receives its own electron. In other words, each element receives an electron. Free radicals are formed as a result of homolytic cleavage.

Covalent Bond Cleavage via Homolytic Cleavage
We used an arrow to depict electron mobility in the diagram above. The arrow employed in this circumstance is known as the fish-hook arrow since it indicates that only one electron is moving.
Heterolytic Cleavage: What Is It?
The production of charged species occurs when the covalent bonds between two atoms break heterolytically, that is, unequally. Heterolytic cleavage is a type of bond breakage in which the electrons are distributed unevenly.

Covalent Bond Cleavage via Heterolysis
We utilised arrows to represent electron movement in the above diagram; a regular arrow indicates that two electrons are being transferred.
Organic Chemistry Reaction Intermediates
The initial product of a subsequent reaction is known as an intermediate. If AB and BC are both present in a chemical reaction, then B is the intermediate for reaction AC. The creation of these intermediates is how organic chemistry reactions happen.
What exactly are carbenes?
Carbenes (H2C) are electron-deficient neutral and reactive entities with six electrons in the outer shell of carbon. We can classify carbenes based on their spin states since they are species with two odd electrons.
Singlet Carbene
The electrons are in distinct orbitals, each with a different spin. In sp2 hybridised orbitals, the electrons are coupled and act as paired electrons.
As the electrons in singlet carbene are antiparallel, the spin state is (2S + 1).
As a result, Spin state = (2×0 + 1) = 1 Carbene Triplet
Both electrons are located in distinct orbitals but have the same spin.
The two electrons in a triplet carbene have the same spin, the spin state is (2S + 1).
As a result, Spin state= (2×1 + 1) = 3.
Singlet and Triplet Carbene Hybridization
Carbene singlet Hybridisation: They have a bent sp2 hybridised form. They feature a 103° bond angle and a 112 pm bond length.
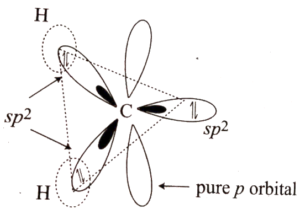
Singlet Carbene Hybridization
Carbene triplet Hybridization: They have a linearly shaped sp hybrid orbital. They have 180° and 103 pm bond angles and lengths, respectively.
Why is the Triplet Carbene more stable than the Singlet Carbene?
Triplet carbene contains less energy than singlet carbene because interelectronic repulsions are stronger in singlet carbene because both electrons are in the same orbital, whereas in triplet carbene the two electrons are in distinct orbitals, resulting in less energy.
What do Free Radicals stand for?
The homolytic breakage of a carbon bond produces free radicals in organic chemistry. The carbon is sp3 hybridised with an odd electron inserted in the p-orbital, and the species generated is planar. They may have a planar structure if the free radical is highly stable.
Free Radicals’ Planar Structure
Carbanions and Carbocations
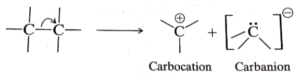
What are Carbanions, exactly?
They are made by cleaving a group bound to carbon heterolytically without removing the connected electrons. This gives the carbon a pair of electrons, giving it a negative charge. Because of the presence of a lone pair of electrons, CH3– is isoelectronic with NH3. It is sp3 hybridised and has a pyramidal structure.
Carbanions and Carbocations are formed.
What are Carbocations ?
Carbocations are classified as cations because they have a sextet of electrons on the carbon-containing positive charge. It possesses a vacant p-orbital and is sp2 hybridised. Planar is the shape. It is usually generated by cleaving a carbon-heteroatom link heterolytically.
Reagents in Organic Chemistry
Reagents are chemicals that are added to organic molecules to cause a specific transformation. In organic chemistry, any generic reaction can be written as:
Product = Substrate + Reagent
The substrate is an organic molecule to which the reagent is added. The reagents are categorised as follows based on their capacity to give or abstract electrons:
- Electrophiles
- Nucleophiles
Electrophiles
Electrophiles are organic chemicals that lack electrons. All species with a positive charge can be considered electrophiles in general. H+, NO2+, CH3+, and Cl+ are other examples.
Electrophiles are neutral substances that are lacking in electrons. Neutral electrophiles include Lewis acids such as AlCl3 and BF3.
Nucleophiles
Nucleophiles are organic reagents that are electron-rich. They are nucleophiles because they seek bonding centres with other nuclei. Negatively charged species might be considered nucleophiles in general. H–, CH3–, and Cl–, for example.
A nucleophile is a neutral molecule having a lone pair of electrons on the heteroatom. For instance, H2O, NH3, and CH3OH.
Types of reaction in Organic Chemistry
Organic reactions take place when organic molecules interact. Organic chemistry reactions are broadly divided into six types. Let’s take a closer look at these various types of reactions and their outcomes.
Substitution Reactions
R-X + Y → R-Y +X
The substrate is R-X, the reagent is Y (which can be electrophilic or nucleophilic), and the leaving group is X. The phrase substitution refers to when one group takes the place of another.
Types of Substitution Reaction:
- Substitution of Nucleophiles ( SN1, SN2, SNi)
Substitution of Electrophiles (SE)
- Aromatic Nucleophilic Substitution (SNAr)
- Reactions of Addition
There are several types of addition reactions:
- Addition of Electrophiles
- Addition of Nucleophiles
- Reactions of elimination
Elimination Reactions
These reactions are the inverse of addition processes, in which a simple molecule (HX, H2O) is removed from the substrate, i.e. a molecule is deleted from the substrate. E1, E2, and E1CB are the different types of elimination reactions.
- Reactions of oxidation and reduction
- Reactions involving pericycles
- Molecular reorganisation.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






