A carboxylic acid is just an organic substance with a carboxyl functional group. These chemicals are primarily found in nature and thus produced by humans. When a carboxylic acid is prepared, they make a carboxylate unpaired electron with structural formula R-COO–that you can use to build a variety of valuable salts, including soap. C=O contains the carboxylic acids that are the most significant functional group. Certain carboxylic acids, including citric acid, lactic acid, or fumaric acid, were created via fermenting, and the majority of these carboxylic acids are helpful in the food business.
These are some of the methods of preparation of carboxylic acids
The primary carboxylic acid preparation method entails the oxidation of several kinds of functional groups. Now let us look at some of the preparation methods of carboxylic acid.
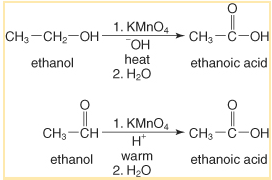
Primary alcohol use in the Preparation
Primary alcohols and aldehydes could transform as carboxylic acids using oxidizing agents, including potassium permanganate (KMnO4 for neutral, acidic, and alkaline media), chromium trioxide (CrO3– H2SO4– Jones reagent), and potassium dichromate (K2Cr2O7– acidic media).
Adding oxidizing chemicals to primary alcohol is oxidized methods of preparation of carboxylic acids. As a result, primary alcohols oxidize to produce aldehydes, then deteriorate again to create carboxylic acids. Potassium dichromate, potassium permanganate, or chromium trioxide are powerful oxidizing agents that can easily convert an aldehyde to carboxylic acids.
Mild oxidisers, on either hand, could only convert primary alcohols as well as aldehydes in a single step. Oxidizing solid agents include manganese dioxide (MnO2) and Tollen’s reagent [Ag(NH3)2+OH-]. As a result, they aren’t capable of oxidizing twice. As a result, mild oxidizing agents are being used to convert aldehydes into carboxylic acids.
It’s essential to remember that acidified oxidizing agents, such as potassium and Jones reagent, create esters in trace levels. As a result, alkaline and neutral substances such as potassium permanganate were appropriate for this carboxylic acid method of preparation.
Preparation based on aldehyde
The previous section described that carboxylic acid could utilize ordinary potent oxidizing agents. Mild oxidizing agents like Tollens reagents [Ag(NH3)2+OH-], as well as manganese dioxide, can create carboxylic acids (MnO2).
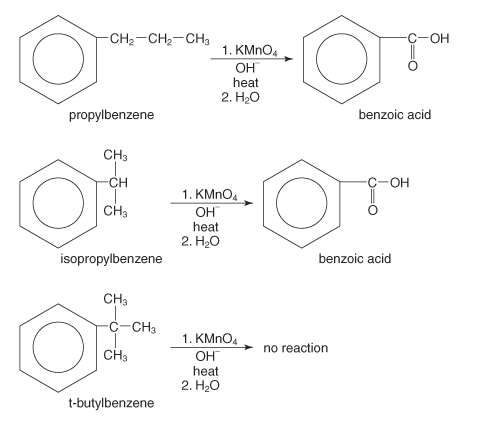
Alkylbenzenes utilize in the preparation process
Alkylbenzenes could be oxidized to produce aromatic carboxylic acids. The rapid oxidation of alkylbenzene compounds with acidic and alkaline potassium permanganate and chromic acid can produce aromatic carboxylic acid compounds. The carboxyl group’s entire side chain gets oxidized, considering the length of the side chain.
These reactions vary depending on whether primary or secondary alkyl groups were present. On the other hand, the tertiary alkyl group is unchanged. Moreover, these oxidizing agents can properly replace alkenes for carboxylic acid preparation.
Preparation with nitriles
When nitriles are hydrolysed, amides are produced. These amides were subsequently exposed to a reaction with an acid catalyst, making carboxylic acids. As in response, H+ or OH- functions as a catalyst. Furthermore, using mild conditions helps in the amide stage reaction’s completion.

Amide-based preparation
In the presence of catalysts, such as H+or OH-, amide is hydrolysed to generate carboxylic acids.
Acyl halides, as well as anhydrides, are used in the preparation
When acid chlorides are hydrolysed with water, carboxylic acids are produced. Acid chlorides may also be rapidly hydrolysed with an acidic base to produce carboxylate ions, which can then be acidified to make the necessary carboxylic acids. In the presence of water, both anhydrides are hydrolysed to create a sufficient acid. We can now conclude.
- Carboxylic acids are made by hydrolysing acid chlorides using water.
- Acid chlorides combine with a base, resulting in carboxylic acid upon further acidification.
- Carboxylic acids are formed whenever acid anhydrides are hydrolysed.
Esters produce carboxylic acids
Carboxylic acids are formed when acidic esters are hydrolysed. On the other hand, the base is hydrolysed to produce carboxylates, subsequently acidified to produce equal carboxylic acids. Likewise, in the method of preparation of carboxylic acids, esters are hydrolysed using mineral acids and alkali.
Grignard reagents preparation
The Grignard reagents reaction produces carboxylic acid. Carboxylic acid salts were generated whenever Grignard reagents reacted to crushed dry ice and solid carbon dioxide. When carboxylic acid salts were acidified with mineral acids, similar carboxylic acids were produced.
As a result, Grignard reagents or nitriles could be made from alkyl halides. The methods help to convert alkyl halides into carboxylic acid equivalents. The resulting carboxylic acid preparation always has one additional carbon atom over equivalent alkyl halides.
Carboxylic Acids Have a Variety of Applications
Carboxylic acids play an important role in our daily life. They are a crucial component in the production of many everyday items. The following are among the most prevalent applications:
- Production of soaps
- It comes in handy in the food business. It’s also used in the manufacture of soft drinks.
- Vinegar is made with this ingredient.
- It is also extremely beneficial in the pharmaceutical sector. It’s a key component in the manufacture of medications like aspirin and others.
- Rubber is another industry that uses it.
- These acids are being used to make dyes, fragrances, and other products.
Conclusion
Carboxylic acids were classified according to the IUPAC nomenclature. Because carboxylic acids invariably end in “-oic acid,” the suffix of this carbon chain is then substituted. The oldest active carbon chain in CH2O2 is methane. I hope you got all the necessary information regarding the carboxylic acid preparation. To better understand the topic, you must go through this information.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






