In 1873, Johannes Diderik van der Waals created the Van der Waals equation. Gasses consisting of point masses that undergo flawlessly elastic collisions are stated by the law of ideal gas. The behavior of real gasses was failed to explain by the Ideal Gas Law. The Van der Waals equation is a modified edition of the Ideal Gas Law. The van der Waals equation helps us to determine the physical state of a real gas, and that’s why the Van der Waals equation was composed. Most importantly, the molecular interaction forces and molecular size is taken into deliberation by the Van der Waals equation. The Van der Waals equation is furthermore referred to as the Van der Waals equation of state.
Body
Definition of the Van der Waals Equation:
An equation about the relationship between the number of real gases, temperature, and volume pressure is known as the Van der Waals equation.
The Van der Waals equation is written for a real gas, that contains ‘n’ moles is:
In which,
- ‘P’ is referred to as the pressure
- ‘V’ is referred to as the Volume
- ‘T’ is referred to as the temperature
- ‘n’ is referred to as the mole of the gas
- ‘a’ and ‘b’ are referred to as the constant specific to each gas.
The Van der Waals equation for a real gas that contains ‘n’ moles can also be written as:

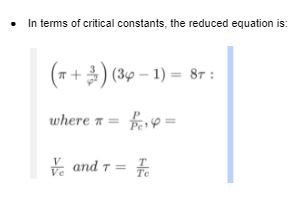
Derivation of Van der Waals equation:
By the Kinetic Theory of Gasses, the deviation of the Van der Waals equation is based on rectifying the volume of the ideal gasses and the pressure. Based on the abilities of the particles, this additional deviation is also used. Anyhow, the same kind of relationship is established by both of the derivations.
Van der Waals equation applied to compressible fluids:
Varying particular volume is contained by the compressible fluids like polymers can be written as:
That is, (p+A)(V−B)=CT
In which,
p is referred to as the: pressure
V is referred to as the: specific volume
T is referred to as the: temperature
And the A, B, C are referred to as the parameters.
Van der Waals Equation Derivation for one mole of gas:
From the non-interacting point particles, let the one mole of gas be formulated.
According to the Ideal Gas Law equation:
That is, PVm = RT
Now, in decreasing the accessible space, assume that the impact of the limited volume of the particles is there in which the available particles are free to move. In this, b is referred to as the co volume or the excluded volume, accordingly, V will be replaced by the ‘b’ ( which is the co volume).
Hence,
We got = P (Vm – b) = RT
or,
i.e., P = RT/(Vm – b)
Now by considering the between particle’s attractive forces. We get,
On the surface area of the molecule, the cumulative net force acts, by pulling it into the container, gets instantly equal to the number destiny as:
i.e., C = NA/ Vm
Further, by a factor relative to the square of the destiny, the forces between the walls are reduced,
The force per unit area is reduced by, that is, a’ C² = a’ (NA/ Vm)² = a/Vm²
Therefore, the net pressure becomes
i.e., P = RT/(Vm-b) – a/Vm²
Or, (P + a/V²) ( V – b) = nRT
Thereupon, by composing nVm equal to V and by writing n for the number of moles, the second form of equation will be provided in results,
And that is = (P + an²/V²) ( V – nb) = nRT
Some merits of Van der Waals equation:
- It can analyze the behavior of ideal gas more accurately than the ideal gas equation.
- For fluids also, the Van der Waals equation is valid.
- For calculating the volume below the critical temperature of the gas, the Van der Waals equation provides us with three various types of volume which can be further used.
Some demerits of Van der Waals equation:
- Over the critical temperature, the Van der Waals equation can provide accurate results.
- The values below the critical temperature are also accepted in the Van der Waals equation.
- The Van der Waals equation ceases to answer during the transition of gasses.
Conclusion:
The behavior of real gasses was failed to explain by the Ideal Gas Law. The Van der Waals equation is a modified edition of the Ideal Gas Law. ‘a’ and ‘b’ are referred to as the constant specific to each gas. Based on the abilities of the particles, this additional deviation is also used. For fluids also, the Van der Waals equation is valid. The values below the critical temperature are also accepted in the Van der Waals equation. Gasses consisting of point masses that undergo flawlessly elastic collisions are stated by the law of ideal gasses. It can analyze the behavior of ideal gas.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



