Introduction
Hydrogen (H) is a combustible vaporous material that is bland, odorless, and colorless. It is a gas that has the most particular synthetic component. The hydrogen molecule has a core that involves a proton with a positive electrical charge of 1 unit. An electron carrying one negative electrical charge has a connection with this core. Under normal conditions, this element acts as a free accumulation of hydrogen particles. Let us take a look at hydrogen introduction and learn about its usage. We shall also go through its properties and an introduction to hydrogen bonding. Besides, we will also go through an introduction to fuel cells.
Physical Properties of Hydrogen
No discussion of hydrogen introduction can be complete without first going through physical properties. Below are the various physical characteristics of hydrogen:
- It is the smallest chemical element because it involves only one proton
- The atomic number of this element is 1
- Its average atomic weight is 1.0079 amu
- It is the lightest known element in existence
- It is the most abundant chemical substance in the entire universe
- Hydrogen exists in vast amounts in stars and gas giant planets
- Monoatomic hydrogen exists on Earth but in rare amounts because of its characteristic of making bonds of covalent nature.
- At standard temperature and pressure, this element displays the qualities of being highly combustible, colorless, tasteless, odorless, non-metallic, and nontoxic
- Hydrogen is also prevalent on Earth in the form of hydrocarbons and water
Isotopes of Hydrogen
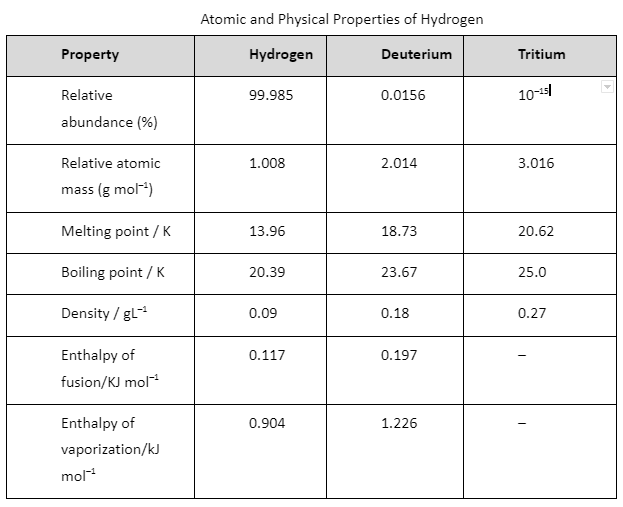
Hydrogen has three isotopes: protium, , deuterium, or D and tritium, or T. These isotopes differ from one another in respect to the presence of neutrons. Ordinary hydrogen, protium, has no neutrons, deuterium (also known as heavy hydrogen) has one and tritium has two neutrons in the nucleus. In the year 1934, an American scientist, Harold C. Urey, got the Nobel Prize for separating hydrogen isotope of mass number 2 by physical methods.
The predominant form is protium. Terrestrial hydrogen contains 0.0156% of deuterium mostly in the form of HD. The tritium concentration is about one atom per 1018 atoms of protium. Of these isotopes, only tritium is radioactive and emits low energy b– particles.
Different forms of Hydrogen :
(a) Based on oxidation Number.
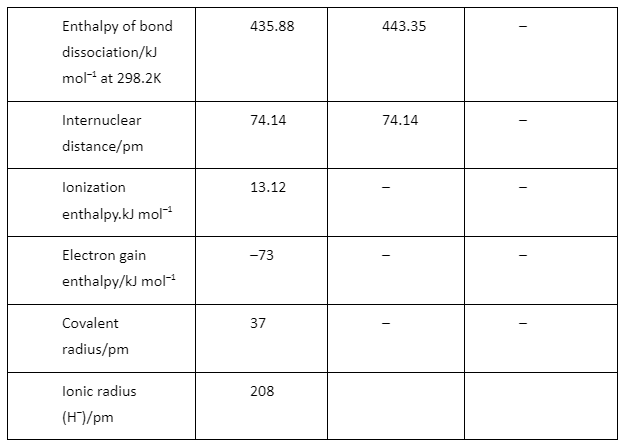
There are three types of hydrogen H+ H– H Proton Hydride Atomic hydrogen Number of electron 0 2 1 Oxidation number +1 –1 0 Formation H ® H+ + e– H + e– ® H– H2 2H(b) Based on reactivity :
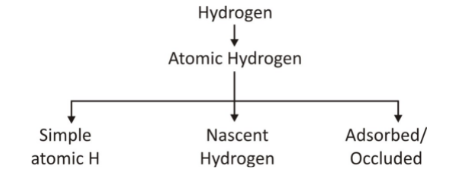
Atomic hydrogen :
(i) Simple atomic hydrogen –
It is formed by simple dissociation of hydrogen.

(ii) Nascent hydrogen –
Hydrogen at the moment of its birth is called nascent hydrogen, which means that it forms at the instant it is known as nascent hydrogen. It is formed only by some specific chemical reaction. (a) Acid + Metals Zn +H2SO4→ ZnSOH4SO4 + 2H (b) Base + element 2NaOH + Be → NaH2SO4BeOH2SO4 + 2H (c) CH2SO4H5OH + Alkali Metal C2H5OH + Na → C2H5ONa + H(iii) Adsorbed/Occluded hydrogens

Adsorbed H is hydrogen present at the outer surface of metal.
Occlusion – The property of metal to absorb any gas is called occlusion.
Reactivity order
Atomic hydrogen > Nascent hydrogen > Molecular hydrogen
(iii) Based on Nuclear spin (Nuclear isomers)
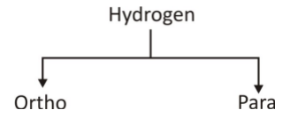
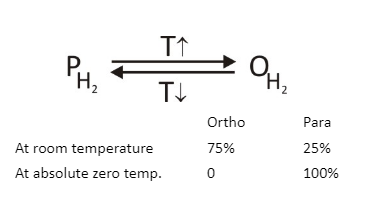
(a) Ortho hydrogen – The molecular form of hydrogen having the same spin of nucleus is called ortho hydrogen.
(b) Para hydrogen – The molecular form of hydrogen having opposite spin of the nucleus is called para hydrogen.
In ortho hydrogen the spin of the nucleus is the same, so they will repel each other & because of this repulsion, internal energy of ortho hydrogen increases. So ortho hydrogen has more internal energy.
Stability of ortho & para hydrogen
Stability of ortho & para hydrogen depends upon temperature condition.
At low temp : para hydrogen is more stable than ortho hydrogen while at high temp ortho hydrogen is more stable than para hydrogen.
PREPARATION OF DIHYDROGEN, H2
There are a number of methods for preparing dihydrogen from metals and metal hydrides.Laboratory Preparation of Dihydrogen
(i) It is usually prepared by the reaction of granulated zinc with dilute hydrochloric acid. Zn + 2H+ → Zn2 + H2 (ii) It can also be prepared by the reaction of zinc with aqueous alkali. Zn + 2NaOH →N2ZnO2 + H2 Sodium zincateCommercial Production of Dihydrogen
The commonly used processes are outlined below: (i) Electrolysis of acidified water using platinum electrodes gives hydrogen.


The production of dihydrogen can be increased by reacting carbon monoxide of syngas mixtures with steam in the presence of iron chromate as catalyst (Fe2O3 and Cr2O3).

This is called the water-gas shift reaction Bosch process. Carbon dioxide is removed by scrubbing with sodium arsenite solution. Presently ~77% of the industrial dihydrogen is produced from petro-chemicals, 18% from coal, 4% from electrolysis of aqueous solutions and 1% from other sources.
Chemical Characteristics
A good understanding of hydrogen requires the study of its chemical properties. Below are the various chemical characteristics of hydrogen:
- The atomic number of this element is 1
- Its density at 20 °C is 0.0899*10 -3 g.cm -3
- The electronegativity, according to Pauling, is about 2.1
- Its atomic mass is about 1.007825 g.mol -1
- It has 3 isotopes
- Its energy of first ionization comes about 1311 kJ.mol -1
- The melting point of this element is – 259.2 °C
- The boiling point of this element is – 252.8 °C
- The Van Der Waals radius of hydrogen is 0.12 nm
- Its Ionic radius is 0.208 (-1) nm
- The electronic shell of hydrogen is 1s1
Uses of Dihydrogen
- The largest single use of dihydrogen is in the synthesis of ammonia which is used in the manufacture of nitric acid and nitrogenous fertilizers.
- Dihydrogen is used in the manufacture of vanaspati fat by the hydrogenation of polyunsaturated vegetable oils like soyabean, cotton seeds etc. . It is used in the manufacture of bulk organic chemicals, particularly methanol.

Lorem ipsum dolor sit amet, consectetur adipiscing elit. Ut elit tellus, luctus nec ullamcorper mattis, pulvinar dapibus leo.
- It is widely used for the manufacture of metal hydrides.
- It is used for the preparation of hydrogen chloride, a highly useful chemical.
- In metallurgical processes, it is used to reduce heavy metal oxides to metals.
- Atomic hydrogen and oxy-hydrogen torches find use for cutting and welding purposes. Atomic hydrogen atoms (produced by dissociation of dihydrogen with the help of an electric arc) are allowed to recombine on the surface to be welded to generate the temperature of 4000 K.
- It is used as a rocket fuel in space research.
- Dihydrogen is used in fuel cells for generating electrical energy. It has many advantages over conventional fossil fuels and electric power. It does not produce any pollution and releases greater energy per unit mass of fuel in comparison to gasoline and other fuels.
Introduction to Hydrogen Technology
As an introduction to hydrogen technology, we must focus on fuel cells. For an introduction to fuel cells, we must understand that hydrogen fuel cells are a clean alternative to traditional combustion energy. This is because these cells are free of emissions. They make use of hydrogen for the production of energy through reactions of an electrochemical nature. These cells are made even more nature friendly when the production of hydrogen takes place through sustainable means.
There can be various types of differences in fuel cell technology. However, there is a certain level of similarity between all of them. All fuel cells are characterized by an anode and a cathode that exist on either side of an electrolyte. In a proton-conducting fuel cell, the feeding of hydrogen takes place at the anode while the use of a catalyst helps produce ions that are positively charged.
The flowing of these ions takes place through the electrolyte to the cathode. This induces a current that results in the formation of electricity. Meanwhile, at the cathode, the feeding of air takes place in the system. This air combines with electrons, hydrogen ions, and the catalyst to form byproducts like water and heat.
In our introduction to hydrogen, we must also learn about the various types of fuel cells. Since their invention in 1838, fuel cells have undergone many changes. As of now, there are three main types of fuel cells which are as follows:
- Proton exchange membrane fuel cells (PEMFCs)
- Molten carbonate fuel cells (MCFCs)
- Phosphoric acid fuel cells (PAFCs)
Introduction of Hydrogen Bonding
Learning about hydrogen bonding is important when studying an introduction to hydrogen. Hydrogen is highly flammable. It reacts with most elements to form hydrides. Moreover, it has a reactive property of eliminating metallic oxides. This results in the elemental state of the metal.
Surfaces of metals whose mixture does not take place with hydrogen result in the creation of stable hydrides.
Molecular hydrogen has the ability to undergo reactions with a large variety of compounds as well as elements. However, the reaction becomes inconsequential at ambient temperatures due to the extremely slow rate. Nevertheless, at high temperatures, the rates tend to become rapid. This results in hydrogen bonding.
Conclusion
Hydrogen (H) is a combustible vaporous material whose main characteristics are being bland, odorless, and colorless. It acts as a free accumulation of hydrogen particles under normal conditions. There are various physical and chemical properties of hydrogen that one must study in an introduction to hydrogen. Learning about Hydrogen Technology and fuel cells is an important part as well. The three main types of fuel cells are – Proton exchange membrane fuel cells (PEMFCs), Molten carbonate fuel cells (MCFCs), and Phosphoric acid fuel cells (PAFCs). Molecular hydrogen is reactive with a wide category of compounds and elements.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



