Every machine around us runs on fuel of some kind. While this fuel is something we can’t imagine our lives without right now, have you ever wondered how this fuel is produced?
When it comes to the breakdown, many substances are involved in the production of fuel. However, one of the most important materials needed to make this fuel is a type of chemical compound, known as alkanes.
By definition, alkanes are defined as the chemical compounds that have Carbon and Hydrogen atoms, C and H respectively. This makes them an example of hydrocarbons.
Introduction to Alkanes
Alkanes are popularly used as Paraffin compounds. Fuels like diesel, kerosene and more are basically alkanes that burn with oxygen to give out energy. All the fuels come from the petroleum industry.
When we talk about organic chemistry, we generally start from alkanes.
Since alkanes are also called saturated hydrocarbons, they only consist of Carbon (C) and Hydrogen (H) atoms. These atoms are bonded by single bonds only, which means that all the atoms only share just one pair of electrons with each other.
The general formula for alkanes is CnH2n+2
As alkanes just consist of a single bond, they can be divided into the following three groups:
- Linear straight-chain alkanes
- Branched Alkanes
- Cycloalkanes
Linear straight-chain alkanes
Linear alkanes are those alkanes that have the carbons bonded together in a chain-like structure.
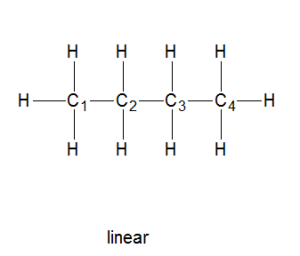
In such compounds, there are two members in a sequence that differ by a few atoms from the other molecule.
Branched Alkanes
Derived from linear alkanes, branched alkanes are differentiated on the basis of having a branched structure with one or more alkyl groups as compared to having a straight chain.
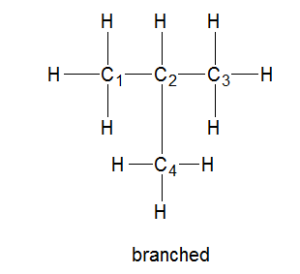
Alkyl groups are groups of carbon and hydrogen atoms attached to an alkane molecule.
Cycloalkanes
Cyclic alkanes (or cycloalkanes) have carbon and hydrogen atoms that are bonded together with single bonds.
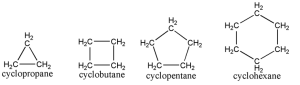
In such bonds, the carbon atoms bond together to form a loop.
Quite similar to cycloalkanes, halogenated alkanes produce a hydrocarbon derivative where one or more halogen atoms are substituted for hydrogen atoms.
Methods of preparation of Alkanes
Since alkanes are colourless and odourless and have different states in different Van Der Waals forces, they are prepared in laboratories through a few techniques. The methods of alkanes preparation include:
From Haloalkanes
Halogenated alkanes (or haloalkanes) are prepared by Wurtz Reaction. By heating alkyl halides with sodium metal in the presence of dry ether, alkanes are obtained.
This reaction is known as the Wurtz reaction and could be used to prepare symmetrical alkanes.
From unsaturated hydrocarbons
This is another method of preparation of alkanes.
Alkanes could also be prepared by the catalytic hydrogenation of unsaturated hydrocarbons. However, it needs to be in the presence of a catalyst ‘Ni’ at 200° C to 300° C.
By the reduction of alkyl halides (RX)
When the haloalkanes (R-X) are heated with reducing agents, alkanes are produced.
By the Reduction of Aldehydes and Ketones
Since aldehydes and ketones could be reduced into alkanes, they are one of the best methods of preparation of alkanes. However, there’s a need for reducing agents like amalgamated zinc and concentrated HCl.
From Grignard’s Reagent
Another common method to prepare alkanes is by hydrolysis of Grignard’s reagent in the presence of ether.
Even though there are other methods of preparation of alkanes like metal carbides or the salts of Carboxylic acid, the above mentioned methods are usually the most common methods of preparation.
Properties of Alkanes
Alkanes, just like most of the compounds, have physical and chemical properties as well.
- Colourless and odourless
- Possess weak Van Der Waals forces of attraction
- Alkanes when having 1-4 carbon atoms are gases, 5-17 carbon atoms exist as liquids and 18 or more exist as solid.
- Alkanes exist as non-polar molecules because of the covalent bonds between C-C and C-H. Along with that, the difference between electronegativities of carbon and hydrogen also add to this behaviour.
- Alkanes have a lower density than water, which increases with the increase in their molecular mass.
- The carbon atoms are sp³ hybridised, meaning they form four sigma bonds with either carbon or hydrogen atoms.
Uses of Alkanes
Alkanes have been used for over a century in a variety of industries and other places. In fact, they are one of the most important raw materials for the chemical industries and a major constituent of gasoline.
Natural gases, an alkanes example, are usually used as fuel, for utilities or even for cooking purposes. This natural gas contains methane and ethane, the byproducts of alkanes.
Along with being the constituents of fuels, the derivatives of alkanes could also be found in plastics of different kinds, cosmetics, and pharmaceuticals.
Butane and propane, when under lower pressure, are liquified. This converts them into LPG which has dozens of applications in our households and industries alike.
Alkanes (C35 precisely) are also used as paraffin wax candles and have lubricative applications as well.
Conclusion
Alkanes are one of the starting points for organic chemistry. Methane (CH4) is one of the simplest alkanes, that has one atom of carbon and four atoms of hydrogen, bonded by a single bond.
The list of alkanes starts from methane (CH4) and continues as Ethane (C2H6), Propane (C3H8), Butane (C4H10), Pentane (C5H12), Hexane (C6H14), Heptane (C7H16), Octane (C8H18), Nonane (C9H20), and Decane (C10H22).
The concept of alkanes is the beginning for your in organic chemistry. The better you understand its fundamentals and formulae, the faster you could solve its questions in your competitive exams.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






