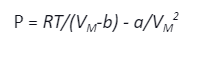Thermodynamics is that branch of physics and chemistry that deals with heat transfer in the given system. It uses certain equations that inter-relate various parameters such as temperature, pressure, volume, etc.
What is thermodynamic equilibrium?
As discussed above, thermodynamics deals with heat in a system. Some state parameters are used to define the system’s state at a given time. When a system is said to be in equilibrium, there is no net macroscopic flow of matter or energy. A system has fixed parameter values at a given time. All these values are interrelated. If any parameter changes, the state of the system also changes. In the case of thermodynamic equilibrium, there is no change in parameters and hence no change in the form of the system.
What are thermodynamic state variables?
To define the state of a system in thermodynamics, we use various parameters. These parameters are called state variables. These include:
- Temperature T
- Pressure P
- Volume V
- Entropy S
- Enthalpy H
- Internal Energy Q
- Mass m
- Density ρ
It must be noted that the state variables do not depend on the path taken to reach that state.
Intensive and Extensive Variables
Extensive Variables are the state variables that exhibit the size of the system. For example, volume is an extensive variable because it helps determine its size.
Intensive Variables are the state variables that do not provide any information about the size of the system but indicate different details on the system. Examples of intensive variables are pressure, temperature, etc.
Equations of state
These equations make it easier to determine the properties of a system. There are certain equations of state. Some of them are:
- Ideal gas law: This is the most fundamental equation that relates pressure, volume, temperature, number of moles, and the universal gas constant with each other. It states that
PV=nRT.
- Lee-Kesler Generalised Correlation: This is used to calculate Z as a function of reduced temperature (Tr) and reduced pressure (Pr). Tr and Pr are computed by dividing the temperature and pressure of the gas by its critical temperature (Tr=T/Tc) and critical pressure (Pr=P/Pc). Another solution is found out using compressibility charts. It is expressed as
PV = ZnRT.
- Van-der Waals Law: This law modifies the ideal gas law and deals with natural gases. Here, the coefficients are determined using empirical relations. The law says that
Where ‘a’ represents the attractive interactions in the gas, and ‘b’ represents the repulsive interactions or the molecular volume of the gas particles. VM represents molar volume or volume per mole and T is temperature..
Solved numerical
Example 1:
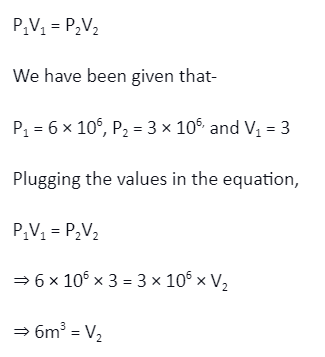
In an isothermal thermodynamic process, initial pressure and volume are measured to be 6 × 106 N/m2 and 3m3, respectively. The pressure of the container is then halved. Find the new volume.
Solution:
For an isothermal process, we know that
Example 2:
Calculate the T of 1 mole of methane (CH4) that occupies 20.0L at 1 atm pressure. The answer should be in Kelvin.
Solution:
Using the ideal gas law,
PV=nRT
T=PV/nR
T = [1.00atm][20.0L]/[1mol][0.082]
T = 244K
Example 3:
At 25°C and 760 mm of Hg pressure, a gas occupies 600 mL volume. What will be its pressure at a height where the temperature is 10°C, and the volume of the gas is 640 mL.
Solution:

Conclusion
This article summarises the topic ‘Thermodynamic State variables and equation of state’. In thermodynamic equilibrium, there is no change in parameters and hence no change in the form of the system. These parameters are defined as state variables, for example temperature (T), pressure (P), volume (V ), entropy (S), enthalpy (H), internal energy (Q), mass (m), density (ρ). Equation of state is a relation between temperature, pressure, moles, and volume that helps determine the properties of a system. In this article we explained three different equations of states. The most used equation is the ideal gas law, PV=nRT. Here, P is pressure, V is volume, T is temperature, n is moles, and R is the universal gas constant.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out