An atom moves freely in a three-dimensional space, but requires energy to do so. This energy comes from the kinetic energy of elementary particles.
The equipartition energy or equipartition theorem is all about the allocation of energy that an atom or a molecule acquires due to its different ways of motion (translational, vibrational, and rotational motion of atoms/molecules).
But before moving further, to understand what the equipartition of energy means, we need to understand the concept of the degree of freedom(d.o.f).
Degree Of Freedom:
Let us imagine an atom in free space. Specifying its location in a three-dimensional coordinate, let’s say, it is the x, y, and z coordinate axis.
This atom can move freely on any of the axes. The movement of an atom from one point to another can be considered as its translational motion. This way, an atom in a free space would have three such translational motions, that is 3 degrees of freedom.
A monatomic (single atom), would have 3 degrees of freedom.
But diatomic molecules (like O2) are free to move in two more directions apart from three translational motions. Such molecules are connected one to one so they can easily rotate about their centre of mass.
Hence, a diatomic molecule would have a total of 5 ways or directions in which it can move.
But when we consider a diatomic molecule to be a non-rigid rotator that means it can also vibrate about its position. This way, it can have more than 5 ways of motion due to the vibrational effect.
This forms the basis for an atom or a molecule, regarding the directions or possibilities of motion it could have.
Such is the concept of degree of freedom which forms the basis of the Equipartition of energy. The degree of freedom of an atom/molecule is the number of ways it can move in different directions.
Equipartition of energy:
Before you go through the concept, it is crucial to first understand what equipartition means.
Equi- means equal and partition- means division.
Together, equipartition means something which is equally divided or distributed.
So by the equipartition of energy, we mean the equal distribution of particles or atoms in different directions.
As said, a particle can have three basic motions, which are translational, rotational, and vibrational respectively. The energy of those moving atoms/molecules is equally distributed over these directions.
Generally, the law states that molecules in thermal equilibrium have the same average energy associated with each independent degree of freedom of their motion.
Kinetic Energy of a monatomic molecule:
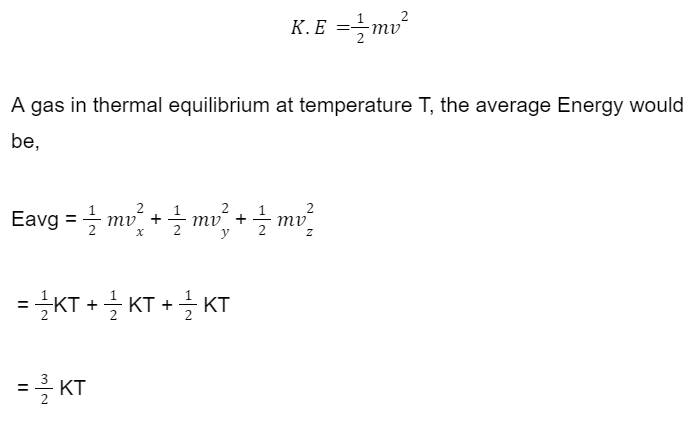
where K = Boltzmann’s constant.
In case of a monatomic molecule, there is only translational motion, the energy allotted to each motion is 1/2 KT
This is calculated by dividing total energy by the degrees of freedom, as follows,
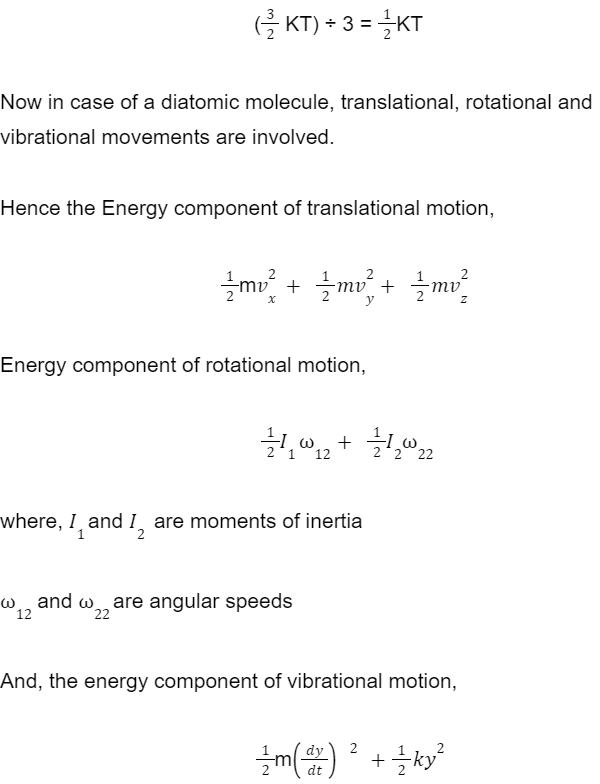
Where k is the force constant of the oscillator and y is the vibrational coordinate (Both kinetic and potential modes are involved here).
Significance of equipartition of energy:
- Ideal gas provides an important application of equipartition of energy.
- With the help of the equipartition theorem, we would be able to derive the formula for Ideal gas as well as Internal energy.
- It can be useful to understand the Brownian motion easily.
- This law can also be used as an astrophysical tool. Using this law along with the virial theorem, we can easily estimate the average temperature of distant stars.
- It is also used to study the current conditions of star formation.
Limitations of equipartition of energy:
The law of equipartition holds only for ergodic systems in thermal equilibrium, which implies that this law of equipartition of energy fails when quantum effects are introduced.
Among three energy distributions, quantum effects dominate vibrational energies for atoms/molecules at very high temperatures, above 1000K.
But this limit is overcome by a newly introduced theory of dynamical systems.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






