Adsorption is the process of adhesion of a chemical species onto the surface of particles. The adsorption is different from absorption. In absorption, a substance diffuses into another through minute pores or holes.
Adsorption is a surface phenomenon, whereas absorption is a bulk phenomenon. In the adsorption process, the surface on which adsorption takes place is called an adsorbent, and the substance that gets adsorbed is called the adsorbate.
The term sorption is used to describe both adsorption and absorption occurring simultaneously. For example, ammonia in water (absorption) and ammonia with charcoal (adsorption).
Types of adsorption isotherms
Temperature has a significant effect on the adsorption process. Hence, isotherms are used to describe adsorption. The method of adsorption is studied through graphs known as adsorption isotherms. The graph between the amount of adsorbate (x) adsorbed on the surface of adsorbent (m) and pressure (P) at constant temperature is shown as
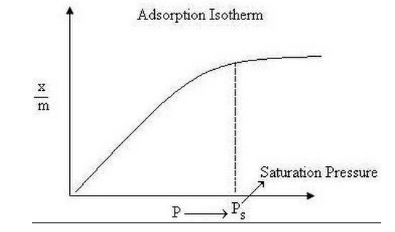
Characteristics of basic adsorption isotherms:
- A limited number of vacancies on the surface of the adsorbent.
- After reaching saturation pressure (Ps), adsorption does not occur anymore.
- At a certain high pressure, all the adsorption sites on the surface are occupied, and a further increase in pressure does not cause any difference in the adsorption process.
- At high pressure, adsorption is independent of pressure.
These different adsorption isotherms can be explained as
Freundlich adsorption isotherm
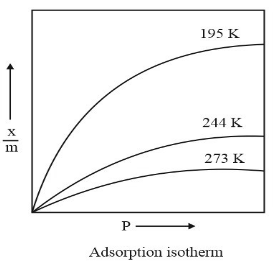
This isotherm estimates the sorption intensity of the adsorbent towards the adsorbate. Dr Herbert Freundlich formulated this theorem in 1909. The relationship between the magnitude of adsorption and pressure can be expressed mathematically by an empirical equation commonly known as Freundlich adsorption isotherm, viz.,
a = kpn
Where a(x/m) is the amount of gas adsorbed per unit mass of the adsorbent at pressure p and k and n are constants depending upon the nature of the gas and the nature of the adsorbent. The value of n is less than one and, therefore, a does not increase as rapidly as p, as evident from the given adsorption isotherms:
Langmuir adsorption isotherm
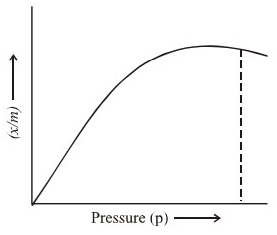
Langmuir investigated the adsorption of gases by solids. He derived the relationship between the magnitude of adsorption (x/m) and P, the equilibrium pressure.
It is represented mathematically as:
(x/m) = (bp/1+ap)
Where a, b are constants. It is known as Langmuir adsorption isotherm. The equation explains the change in the magnitude of adsorption (x/m) with pressure in all adsorption processes. Langmuir adsorption isotherms can be shown as
Types of adsorption chromatography
We know that adsorption chromatography is the oldest type of chromatography technique. This technique has two phases, i.e., a mobile phase and a stationary phase. The mobile phase is either in liquid or gaseous form, and the stationary phase is in the solid form.
In the adsorption chromatography, the mobile phase is adsorbed onto the surface of a stationary solid phase. The equilibration between the mobile and stationary phase accounts for the separation of different solutes. Adsorption chromatography is the separation, purification and identification of the active constituents. An adsorbent is a porous substance that adsorbs substances on its surface by intermolecular forces. Some commonly used adsorbents are silica gel, alumina, and so.
There are different kinds of adsorption chromatography. These are explained as
Thin-Layer Chromatography
In thin-layer chromatography, the mobile phase moves over an adsorbent. The separation of components is accomplished by applying thin layers of adsorbent to solid supports. By this method, the solvent migrates along with the powder by moving along the glass plate as the powder spreads on it.
Paper chromatography
In this technique, the solid surface of the paper is the stationary phase, and the liquid phase is the mobile phase. Paper chromatography refers to making a solution pass through a stationary phase of paper sheets or strips used as the adsorbent. This method is used to separate coloured chemicals or substances.
Column chromatography
Column chromatography is one of the most useful methods for separating and purifying both solids and liquids. This is a solid-liquid technique in which the stationary phase is a solid and the mobile phase is a liquid.
Detection is carried out by adsorption column chromatography, packing of the adsorbent in a glass column followed by a solvent, the mobile phase, moving slowly through the packed column.
Using a solvent as a mobile phase is what eluents do.
As a compound moves rapidly through the column, the mobile phase will attract it, and once it has passed through it, the compound will be dissolved in or eluted from the eluent. The faster the compound passes through the column, the more strongly attracted it is to the stationary phase.
Gas-Solid chromatography
Gas-solid chromatography is used for solutes that have less solubility in the stationary phase. Gas-solid chromatography is not used widely because this type of chromatography technique has a very limited number of stationary phases available.
Conclusion
Adsorption is a widely used process to separate mixtures. The adsorption technique is used in many daily activities and manufacturing purposes. Adsorption isotherms are important for researchers dealing with environmental protection and adsorption techniques. Adsorption chromatography is the oldest type of chromatography technique. There are different kinds of adsorption chromatography techniques and all these techniques are based on adsorption phenomena. Hence, adsorption is important in analytical industries also.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out




