What is Atom?
The word ‘atom’ has been derived from the Greek word ‘a-tomio’ which means ‘uncuttable’ or ‘non-divisible’An atom is the smallest unit of ordinary matter that can be used to make a chemical element. Every solid, liquid, gas, and plasma is composed of atoms that were either neutral or ionized.
Dalton’s atomic theory(1808), regarded the atom as the ultimate particle of matter.
Atomic Structure-
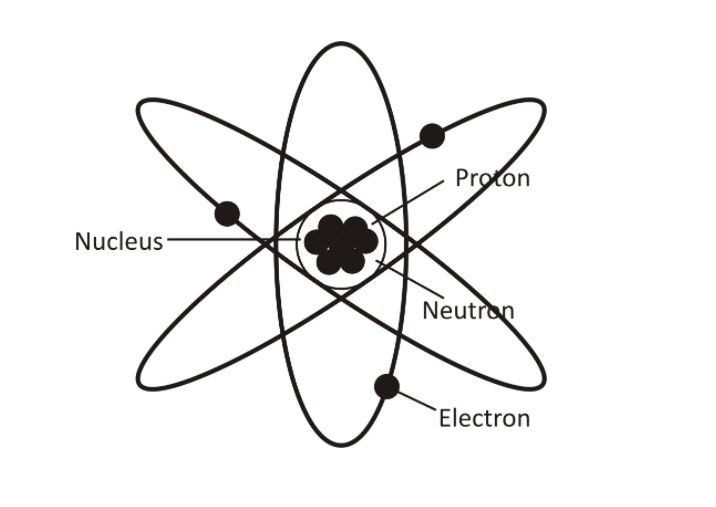
The atomic structure of matter is mostly composed of protons, electrons, and neutrons.
It has a nucleus (center) that contains protons (positively charged) and neutrons (neutral). Electrons, which are negatively charged particles, circulate around the nucleus. An element’s atomic number describes the total number of protons in its nucleus.
Atomic Model-
To explain the distributions of these charged particles in an atom, various atomic models have been developed. Scientists such as John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr contributed significantly to the field.
Dalton’s Atomic Theory:
All substance, according to John Dalton, is composed of atoms that are indivisible and indestructible. He also noted that while all atoms of the given elements are the same, atoms of different elements differ in size and mass.
The postulates of this theory may be stated as follows:
(i) All matter is composed of extremely small particles known as atoms.
(ii) Atoms are indestructible particles that cannot be generated or destroyed during a chemical reaction.
(iii) The weights and chemical compositions of atoms of different elements vary.
(iv) Atoms combine in the ratio of small whole numbers to form compounds.
Limitations of Dalton’s atomic theory :
- (i) Atoms of the same or different types tend to join to form a new group of atoms.
- (ii) Hydrogen and oxygen gases, for example, exist in nature as two-atom groups.
- This implies that the lowest unit capable of independent existence is a group of atoms instead of an atom.
- (iii) The discovery of subatomic particles such as electrons, neutrons, and protons has made the atom no longer indivisible.
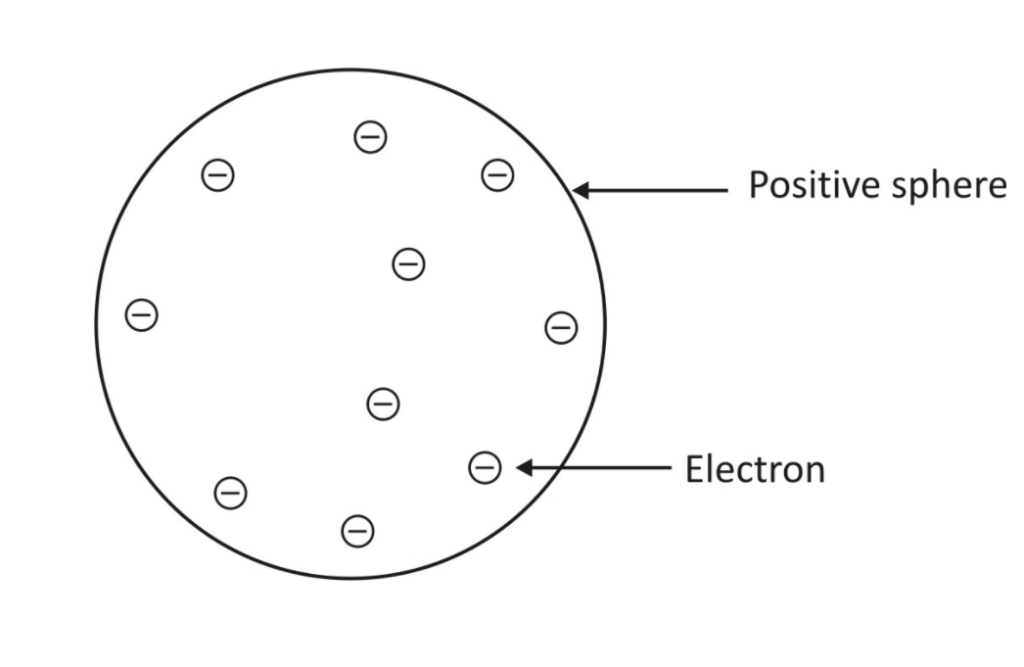
Thomson Atomic Model: (Plum Pudding Model)
- Thomson’s atomic study suggests that an atom resembles a positively charged sphere with electrons existing within the sphere.
- Because the magnitude of the opposite charges is equal, an atom is electrically neutral.
- It resembles both a spherical plum pudding and a watermelon. It resembles plum pudding because the electrons in the model mimic the seeds contained in a sphere of positive charge, similar to a melon spherical pulp.
Limitations of Thomson’s Atomic Model
- Thomson’s atomic model does not explain how the positive charge on the electrons inside the atom is maintained.
- It also failed to explain the integrity of an atom.
- The nucleus of an atom was not mentioned in the hypothesis.
Rutherford nuclear model-
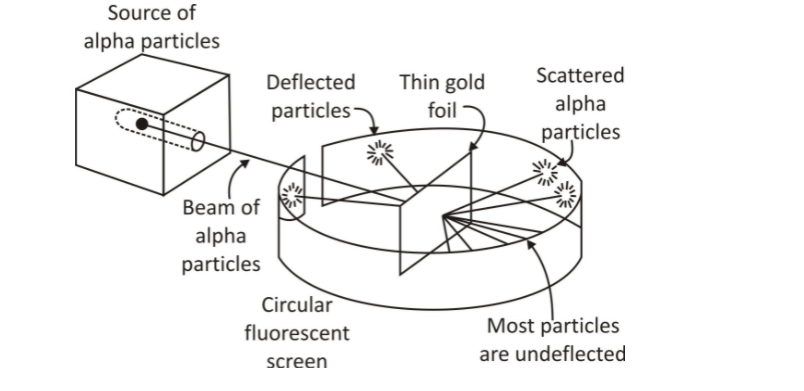
Rutherford and his students (Hans Geiger and Ernest Marsden) performed the gold foil scattering experiment.
Structure:
A thin gold foil sheet.
A stream of high-energy α–particles from a radioactive source was directed at a thin foil (thickness∼100 nm) of gold metal.
The thin gold foil had a circular fluorescent ZnS screen around it. Whenever α–particles struck the screen, a tiny flash of light was produced at that point.
According to Thomson’s model of the atom, the mass of every gold atom in the foil should have been distributed uniformly across the entire atom. The particles were predicted to slow down and shift directions only a little as they traveled through the foil.
Observation-
(i) most of the α– particles passed through the gold foil undeflected.
(ii) a small fraction of the α–particles were deflected by small angles.
(iii) a very few α– particles (∼1 in 20,000) bounced back, i.e., were deflected by nearly 180°.
Conclusion-
(i)The majority of the atom’s region is unoccupied since the majority of the particles passed through the foil undeflected.
(ii) A few positively charged alpha-particles were deflected, indicating that the positive charge of the atom is not distributed evenly throughout the atom. The positive charge must be concentrated in a limited volume in order to resist and deflect positively charged – particles.
(iii) Calculations revealed that the nucleus occupies a negligibly little volume in comparison to the entire volume of the atom. The radius of the atom is approximately 10–10 m, while the radius of the nucleus is approximately 10–15 m.
Atomic Nucleus: All atomic nuclei are composed of nucleons, with the protons in the nucleus determining the compound and the number of neutrons determining the different isotopes of each chemical element.
The composition of the atomic nucleus gives us lots of information about the element it represents.The atomic number is determined by the number of protons contained within the nucleus.
The composition of the atomic nucleus gives us lots of information about the element it represents.The atomic number is determined by the number of protons contained within the nucleus.
Hydrogen has the simplest nucleus, which is only a single proton.
The element hydrogen contains three isotopes: hydrogen, deuterium, and tritium.
They each have one proton (Z = 1) but differ in the number of neutrons they have. Hydrogen has no neutrons, while deuterium has one and tritium has two.
They each have one single proton (Z = 1) but differ in the number of their neutrons. Hydrogen has no neutrons, deuterium has one, and tritium has two neutrons.
The isotopes of hydrogen have mass numbers of one, two, and three respectively. Their nuclear symbols are therefore 1H, 2H, and 3H
The atoms of these isotopes have one electron to balance the charge of the one proton.
Because an atom is so small, its mass is proportionally little as well. A standard measure of mass, such as a Kilogram (kg), cannot be used to weigh something as small as an atom, thus scientists devised a new unit of mass to solve this obstacle. It is known as the Atomic Mass Unit (u). It is referred to as Carbon-12, and one Atomic Mass unit is equal to one-twelfth the weight of one Carbon-12 atom.
1 u = one atom of C-12/ 12 = 1.992647 x 10-26/ 12 kg |
1 u = 1.660539 x 10-27 kg |
This is the mass of a hydrogen atom. Except for a few elements, most of them are whole multiples of the weight of the Hydrogen atom.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out