Introduction
Hydrocarbons that have a double bond between any of two adjoining or adjacent carbon atoms are called alkenes. Alkenes are formed in such a way that the hydrogen atoms in there are always the double of the carbon atom in any given chemical compound. a homologous bond series is formed by the alkenes, hence their general formula is CnH2n. Ethene is the simplest example of alkene that contains only a single double bond into it. It’s structural formula is represented as C2H4.
It is important to have a thorough knowledge of the concepts of alkenes as it not only plays a significant role in our day-to-day lives but also has various industry uses. From an exam point of view Preparations of alkenes is a very important chapter as every year it has been observed that this particular chapter is something that constantly occurs in competitive examinations. Go through the following notes on Preparations of alkenes for a better understanding and scores.
Methods of preparation of alkenes
Several preparation methods of alkenes are there. Some of the most common ones are listed below. Let’s have a look-
Method of Preparing Alkenes From the Alkynes
Unlike alkenes, alkynes are termed as hydrocarbons having the triple bond into the two different adjoining carbon atoms in their respective chemical compounds.
In this section, we’ll discuss the preparation method of alkenes from alkynes. This reaction is carried out in palladised charcoal presence through a process of hydrogenation. The charcoal in this process is moderately deactivated so that the whole preparation could be carried out successfully.
To stop this reaction of preparing alkene from alkyne through hydrogenation, Lindlar catalyst (calcium carbonate coated with palladium) deactivated with the help of lead acetate is used.
NOTE: Alkenes formed through the above mentioned method will tend to have a more cis-form. If you wish to achieve a trans-shaped alkene, you will be required to react with these alkynes in the presence of liquid ammonia, with sodium.
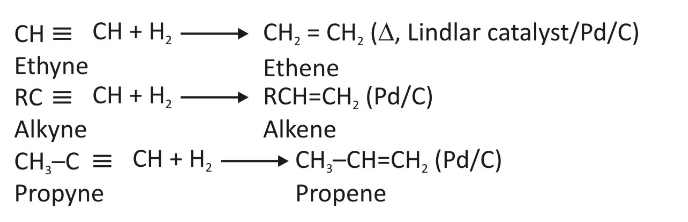
Method of Preparing Alkenes From the Alkyl Halides
Alkyl halides are formed from the Alkenes and are prepared through a method of dehydrogenation.
To prepare alkenes from alkyl halides, the halide needs to be heated in a high temperature that too when the alcoholic KOH is present. This alcoholic KOH can be obtained by dissolving potassium hydroxide in the alcohol.
As soon as the response occurs, a single halogen acid molecule is removed that gives rise to a double bond. The price at which the reaction will take place is determined by an alkyl group and the connected halogen atom. This is likewise called the 1,2 elimination, because the halo atom is released from alpha carbon at the same time as hydrogen atom gets released from beta carbon.
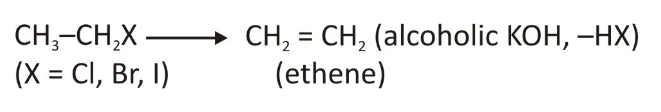
Method of Preparing of Alkenes From the Vicinal Dihalides
In the vicinal dihalides, two halogen agencies are connected to two adjacent carbon atoms placed in the compound.
In case the dihalide gets reacted with the zinc or the sodium iodide into the acetone, the halogen agencies attach to shape a compound with sodium or zinc that leads to the formation of the double bond. This can be termed as dehalogenation.

Method of Preparing the Alkenes From the Alcohols
Everytime, an alcohol is reacted with the focused sulphuric acid, the water molecule gets removed that forms a double bond or an alkene. As the water molecule gets eliminated when the acid is present, the response is often termed as the alcohol’s acidic dehydration.
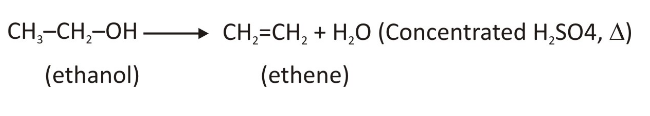
Facts to keep in mind
- Be it liquid, gas, or solid, the Alkenes exist in all the 3 states. If looked closely, the first three alkenes are in the gaseous state , 4 to 14 carbon atom alkanes are found in liquid state.
- They are not water soluble because of the lifestyles of susceptible van der Waal forces.
- On the other hand, Alkenes can be termed as soluble in benzene or acetone that are known as natural solvents.
- If the molar mass of an alkene is higher, its boiling point will also be higher. Therefore, the better alkenes consist of a high boiling point.
- Since alkenes are unsaturated even in nature, they are exceptionally reactive compounds. These reactions mostly occur on the website of a double bond. It is possible for them to undergo oxidation or addition reactions without facing any difficulty.
Conclusion
Preparations of alkenes class 11 is a very important chapter from an exam point of view as it explains the important concepts of various preparation methods of alkenes that is not only going to help you in the entrance or school examinations but also if you decide go in a chemical field, having a thorough knowledge of this chapter will give an edge in your field .
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






