In the case of the p-block element, the last electron takes entry in the p orbital. It includes elements 13, 14, 15, 16, and 17. There can be six electrons all total in the p orbital. P subshell has 3 degenerate orbitals and each orbital can provide accommodation of 2 electrons. The inner part of the p orbital differs and is generally responsible for the physical characteristics of the elements. The general configuration of p block elements is ns (1-2) np (1-6). Due to this atomic configuration, the chemical bonding and molecular structure also differ to a huge extent. The oxidation state also differs a lot due to this variety of electrons present in the p orbital.
Group 13 elements- Boron Family:
The atomic structure of the Boron family is ns2np1. The elements present in the boron family are boron, aluminum, gallium, indium, and thallium. It is easier for the compounds of this family to lose 1 electron rather than accepting 5 electrons to attain the octet. Two types of oxidation states are present in this family.
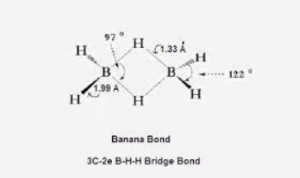
BH3 shows dimers and forms B2H6. The reason behind this is Boron is highly electron-deficient and its electronic configuration is 1s2 2s2 2p1. Due to its vacant p orbital, the electron of hydrogen is shared by two orbitals of two boron atoms and a hydrogen atom. This molecular structure is known as 3 centered 2 electron bond or banana bond. Each B-H-B is highly electron deficient. This is the main reason behind this kind of molecular structure. Two boron atoms exist on the same plane and are sp3 hybridized. At room temperature, this structure of diborane is highly unstable.
The common name of Borax is Na2B4O7. 10H2O. The compound has two tetrahedral units with molecular structure BO4 along with two structures of BO3. Each of the BO4 shares its oxygen atom with the BO3 unit and the second oxygen atom with the other BO4.
Group 14 elements- Carbon Family:
The atomic configuration of the carbon family is ns2 np2. The elements of the carbon family are carbon, silicon, germanium, tin, lead, and flerovium. All elements generally form covalent compounds. The reason behind this is losing four electrons or gaining four electrons for the completion of the octahedral structure is quite difficult.
The structure of benzene consists of six carbons and has a cyclic structure. The molecular orbital structure of benzene consists of a planar hexagonal structure. All the carbon atoms are sp2 hybridized in the structure. One of the p orbitals shows head-on overlapping which forms the sigma bond. The molecular orbital structure of benzene also consists of p orbitals perpendicular to the plane which shows the side overlapping. This side-wise overlapping is responsible for its π- bond. Due to this molecular orbital structure of benzene, it is considered an aromatic hydrocarbon.
Zeolite is an alumina-silicate structure that occupies a lot of water molecules inside the molecules in it. The molecular structure of zeolite consists of a three-dimensional framework that has a tetrahedral structure consisting of oxygen atoms shared by two tetrahedral. The presence of aluminum in the tetrahedral creates an imbalance and that’s why the presence of other metals is required.
Group 15: Nitrogen Family:
The electronic configuration of this family is ns2 np3. This configuration affects the chemical bonding and molecular structure of the compounds formed by these elements. Elements can lose 5 electrons or can gain 3 electrons. Nitrogen forms a tetrahedral structure and can form triple bonds as well.
Conclusion:
There are a variety of structures present in this p block because of its molecular structure as well as electronic configuration. Borane shows dimers and forms diborane because of high electron deficiency. Borax has a tetrahedral structure. In the case of the carbon family, graphite and diamond both are made of carbon but have different chemical properties just because of their structure. Benzene has a cyclic structure with six carbons, each carbon atom is an sp2 structure. The carbon family generally shows a covalent structure. The nitrogen family has 5 electrons in their outermost shell and can release 5 electrons and accept 3 electrons as well. This difference in the molecular structure of the p block is the reason for the different physical and chemical properties of different compounds.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






