Molecular orbitals and valence bond theory are the base theories of quantum chemistry. This chapter contains all the theory portions with diagrams, graphs, and some numerical related to molecular orbital theory. Once you are good at essential concepts, you can solve various problems and logic. If you want to score good marks in examinations, read these notes before appearing for the exam. It is one of the introductory chapters with many applications in upcoming lessons.
What are Molecular Orbitals?
Molecular orbitals are described as the function describing the wave nature of electrons present in a molecule. These molecular orbitals are made after the hybridization process.
Types of Molecular Orbitals
As per the molecular orbital theory, certain types of molecular orbitals are there. These are mentioned below-
Anti Bonding Molecular Orbitals
In the anti-bonding molecular orbitals, the density of electrons is concentrated behind the nuclei of two atoms. This means that two atoms are being pulled away from each other. Hence it results in the weakening of the bond between them.
Non-Bonding Molecular Orbitals
Due to the lack of symmetry in two bonding atomic orbitals, the molecular orbital formed from their interaction has no negative or positive interactions with each other. This doesn’t affect the bond between them.
What is Molecular orbital theory?
The molecular orbital theory is a method that is used to describe the structure of electronic molecules. It is based on quantum mechanics. The theory was proposed in the 20th century. According to molecular orbital theory, an electronic molecule is not bound under any chemical bonds, but they are treated as moving under the influence of the atomic nuclei. As per quantum mechanics, molecular orbitals are defined as electrons’ spatial and energetic properties.
Linear combination of atomic orbitals (LCAO) method
According to the linear combination of atomic orbitals method, every molecule has a set of molecular orbitals. It also proves that the molecular orbital wave function, which is denoted by ψj, can be written as the following equation:
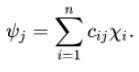
There are some requirements that atomic orbital combinations need to fulfill to be suitable as approximate molecular orbitals. These are mentioned below-
Atomic orbitals must be at the same energy levels to combine as molecular orbitals.
Atomic orbitals must overlap within their space. Molecular orbitals cannot be formed if they have a large distance between them. They can’t combine to form molecular orbitals.
The atomic orbital combination must have accurate symmetry. Molecular orbitals of the correct symmetry can easily be formed by using symmetry-adapted linear combinations.
Conditions for Linear Combination of Atomic Orbitals
Let us explain the above conditions briefly-
Same Energy of Combining Orbitals
Atomic orbitals must be at the same energy level to enable molecular orbitals. This ensures that the 3d orbital has the capabilities to combine with another 3d orbital of another atom, so long as they have the same energy level, but 2p and 1s will never combine with one another as they don’t have the same energy level.
Same Symmetry about Molecular Axis
It means that some orbitals must be symmetric to ensure the molecular axis combines correctly. For example- all the sub-orbitals of 3d have the same energy levels, and yet, the orbital of 3dz can only combine with another 3dz orbital but cannot combine with the 3dx and 3dy orbital. This is because they have a different molecular axis.
Proper Overlap between Atomic Orbitals
To form a molecular orbital, atomic orbitals must be overlapped adequately. If they have a large distance, they can’t combine together.
Features of Molecular Orbital Theory
Atomic orbitals need to overlap with each other to form molecular orbitals. When they form entirely molecular orbitals, the identity of atomic orbitals is wholly lost.
The shape of molecular orbitals entirely depends on the shape of atomic orbitals that are combined.
The electrons in the molecules have new energy levels, which are known as molecular orbitals.
The number of atomic orbitals combined determines the number of molecular orbitals.
The molecular orbital determines the probability of finding the electrons in a molecule.
According to the Molecular Orbital Theory, electrons are filled up according to some rules which are mentioned below-
Aufbau’s principle says that molecular orbitals are filled in the increasing order of their energy.
Hund’s rule of maximum multiplicity: It says that the pairing of electrons cannot take place until the orbitals are singly occupied.
Pauli’s exclusion principle: According to it, no two electrons present in a molecule can have the same set of quantum numbers.
Characteristics of Bonding Molecular Orbitals
The probability of finding the electron in the molecular orbital is more significant than atomic orbitals.
The bonding molecular orbital has lower energy than that of atomic orbitals, which is the result of attraction that leads to greater stability.
The electrons present in the bonding molecular orbital are responsible for their attraction.
They are represented by σ, δ, and π.
Characteristics of Anti-bonding Molecular Orbitals
The probability of finding the electron is less in the anti-bonding molecular orbitals.
The electrons present in the anti-bonding molecular orbital are responsible for the repulsion between them.
The anti-bonding molecular orbitals have higher energy due to the repulsion between their electrons. It lowers its stability.
They are represented by σ∗, δ∗, π∗
Conclusion
Molecular orbitals are formed when two or more atomic orbitals satisfy specific properties. These conditions are mentioned above. This is all you need to know about molecular orbital theory. We discussed everything from theories to diagrams.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






