In the context of surfaces, adsorption refers to the process by which molecules are transferred from one fluid bulk to another on a solid surface. This might happen as a result of physical forces or as a result of chemical bonding. Usually, it is reversible (the reverse process is referred to as desorption); therefore, it is responsible not only for the removal of compounds but also for the release of substances. In the majority of circumstances, this process is characterised at equilibrium by a set of equations that quantify the amount of substance attached to the surface given the concentration of the substance in the fluid present in the fluid. Isotherms (the most renowned of which are the Langmuir and Freundlich equations) are used to describe the behaviour of adsorption equations because their parameters are dependent on temperature, which is one of the most important environmental elements that affect adsorption. As a key activity in ecology, it regulates the exchanges between the geosphere, water column, and atmosphere; it is responsible for the transfer of compounds in ecosystems, and it is the initiator of many other essential processes such as ionic exchange and enzymatic reactions.
The Amount of Adsorption Obtainable
The amount of an adsorbate that is adsorbed per unit mass of an adsorbent is referred to as the extent of adsorption in this context.
As a result, a = x/m
Where a denotes the amount of adsorption
x is the amount of gas that has been adsorbed.
m is the mass of the adsorbent.
The following are the factors that influence adsorption:
Temperature
Because adsorption is an exothermic process, according to Le Chatelier’s principle, when a certain pressure is applied, a low temperature is advantageous for adsorption. When the temperature of the adsorbent is raised, adsorbate molecules are removed from the adsorbent, a process known as desorption. As a result, the rate of adsorption decreases as the temperature increases. This is true for both physical and chemical adsorption. Because of the high energy of activation in chemical adsorption, the extent of adsorption increases initially and then declines as the temperature increases even further in this case.
Pressure
Adsorbent volume decreases when gas is adsorbed on the surface of an adsorbent, indicating that the gas has been absorbed. As a result, according to Le Chatelier’s principle As long as the temperature remains constant, the Following low pressure:
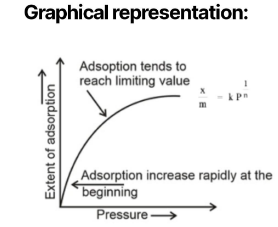
at low pressure 1/n = 1
.. x/m ∝ P
Consequently, at low pressure, the degree of adsorption is precisely proportional to the pressure, and so, at low pressure, the graph is a straight line (see Figure 1).
At medium pressure
At medium pressure 0 <1/n < 1
i. e, x/m ∝ p1/n
In other words, at medium pressure, the extent of adsorption grows less fast with increasing pressure, and hence the graph for medium pressure is shaped like a curvature.
At high pressure
When the pressure is high, 1/n = 0.
x/m = a constant
In this case, the extent of adsorption is independent of pressure, and the graph is therefore parallel to the pressure axis at high pressure.
Nature of Adsorbent
Because adsorption is a surface phenomenon, the number of adsorption increases according to the amount of surface area of the adsorbent. The higher the surface area of an adsorbent, the finer the surface division or the rougher the surface texture. as a result of which the adsorption will be larger. Metal catalysts in finely divided form, colloidal form, rough surfaces, and activated adsorbent provide a greater surface area than other forms of metal catalysts.
Chemisorption is more selective and preferred than adsorption. Gas will only be chemisorbed on a solid if the solid has a wide surface area that can accommodate the gas. For example, nickel can absorb hydrogen gas but not iron, but iron cannot. Adsorbents should have a chemical composition that allows them to cause chemisorption of adsorbate on them.
Nature of Adsorbate
As a result of stronger van der Walls forces, it has been discovered that more easily liquefiable and highly water-soluble gases are adsorbed more readily by solids when the adsorption of gases by solids is investigated. To do this, ammonia, hydrogen chloride, chloride, and sulphur dioxide are adsorbed at a higher rate than hydrogen, nitrogen, and oxygen
When it comes to physical adsorption, the amount of adsorption is determined by the boiling point of the gases involved. Gases are more readily adsorbed on solids than they are on liquids.
The concentration of Adsorbate
Generally, when liquid is adsorbed on solid, the extent of adsorption is greater at higher concentrations of adsorbate, as long as the temperature is maintained at a constant level. The concentration of adsorbate has a comparable impact to that of pressure in terms of its effectiveness.
Adsorption Isotherm
Adsorption isotherms are defined as the relationship between the extent of adsorption (the amount of a substance adsorbed per unit mass of an adsorbent) and the equilibrium pressure or concentration at a constant temperature under uniform conditions. At a fixed temperature and pressure, it is a curve generated by graphing the extent of adsorption (the amount of a substance adsorbed per unit mass of an adsorbent) versus the equilibrium pressure or the concentration of the substance adsorbed.
Freundlich Adsorption Isotherm
Adsorption isotherms are curves that are created by graphing the extent of adsorption (the amount of a material adsorbed per unit mass of an adsorbent) against the equilibrium pressure or concentration while maintaining a constant temperature.
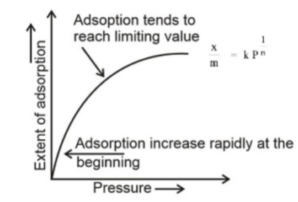
It is known as the “Freundlich adsorption isotherm” since it was proposed by Freundlich and is an empirical equation for the variation of gas adsorption with pressure at a constant temperature over a narrow range of pressure. The equation is as follows in terms of mathematics:
X/m = kP1/n or x/m=kC1/n
Where x denotes the mass of adsorbate (gas) that has been adsorbed.
m is the mass of the adsorbent (solid).
P represents the equilibrium pressure of the adsorbate.
The concentration of adsorbate in solution at equilibrium is denoted by C.
In this equation, the variables ‘K’ and ‘n’ are constant, and the variable ‘n’ is less than one.
Conclusion
It is the process by which a substance (referred to as the adsorbate or the sorbate) is accumulated on the surface of a solid (adsorbent, or sorbent). The adsorbate can exist in either a gaseous or a liquid state. It is unsaturated forces at the solid surface that act as a driving force for adsorption and have the potential to establish bonds with the adsorbate. Adsorption processes that take place on cell membranes stimulate a wide range of important chemical reactions while also causing changes in surface tension and cell consistency, among other effects. Adsorbed drugs and poisons can exert their effects on cell surfaces because of their placement on the cell surface. Selective adsorption may be related to a specific activity. Specifically, the surface area of the adsorbent is directly proportional to the extent of adsorption; that is to say, the bigger the surface area of the adsorbent, the greater is the amount of adsorption. The surface area of a powdered solid adsorbent is determined by the size of the particles in the adsorbent. The higher the surface area of a particle, the smaller the particle size.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






