In 1916 Kossel and Lewis achieved an explanation based upon the notion of electrical configuration of noble gases regarding why atoms unite to form molecules. Atoms of noble gases have hardly any inclination to mix with one other or with atoms. This suggests that these atoms should be possessing stable electronic structures.
Due to the stable arrangement, the noble gas atoms neither have any inclination to receive nor lose electrons and, consequently, their combining capacity or valence state is zero. They are so inert that they even need not form diatomic molecules and live as monatomic gaseous atoms.
Octet Rule
All atoms other than noble gases contain fewer than eight electrons in their outermost shells. In other words, the outermost shells of these atoms do not have stable structures. Therefore, they mix with each other or with other atoms to produce stable noble gas electronic states. These elements perform electronic re-arrangements to obtain stable noble gas structures. Therefore,
“The inclination or drive of atoms of diverse elements to acquire stable form of 8 electrons in their outermost shell, is the source of Chemical combination” and
“The concept of obtaining a maximum of eight electrons in the valence shell of atoms, is termed octet rule.”
Lewis proposed simple symbols to indicate the electrons residing in the outer orbit of an atom, these electrons are called valence electrons. These symbols are called Electron Dot Symbols and the structure of the compound is known as Lewis Dot Structure.
Condition for writing the Lewis dot structures of molecules
Lewis Structures and Covalent Bond requirement for writing the Lewis dot structures of molecules Conditions for writing the electron dot (or Lewis) structures of covalent compounds are:
- Sharing of an electron pair between the atoms leads to the bond formation.
- At the moment of bond formation, every bond consists of two electrons which are provided by each of the combining atoms.
- When the combining atoms form a bond, due to sharing of electrons they attain the stable outermost shell noble gas configurations. In other words, octets of both the atoms become fulfilled.
Electron dot (or Lewis) structures of covalent compounds are written in line with octet rule. As per this, all the atoms in the formula will have a total of 8 electrons in their valence shell excluding the Hydrogen atom. Hydrogen will have just two electrons since only two electrons complete its initial shell as in helium. Thus the elements of group 17 such as Cl would consider sharing one electron to attain stable octet; the elements of group 16 such as O and S might share two electrons; the elements of group 15 would share three electrons and so on.
Types of Covalent Bonds
- Single covalent bond formed when two atoms share one electron pair and is symbolized by one dash (–)
- Double covalent bond when two atoms share 2 electron pairs and is indicated by two dashes ( = ).
- Triple covalent bond formed when two atoms share 3 electron pairs and is symbolized by three dashes ( ≡ ).
Examples of Covalent Bonds
Oxygen Molecule
In the creation of an oxygen molecule, each oxygen atom possesses six electrons in the valence shell and needs two electrons to complete its octet. Therefore the atoms donate two electrons each for sharing to create an oxygen molecule, two electron pairs are shared and consequently it’s a double bond between the two oxygen atoms.
Nitrogen Molecule
In the synthesis of a nitrogen molecule each of the two nitrogen atoms possessing five valence electrons, contributes three electrons to create three electron pairs for sharing. Thus, a triple bond is established between the two nitrogen atoms.
Ethylene Molecule
In ethylene, every carbon atom exchanges two of its valence electrons with 2 hydrogen atoms and remaining two electrons with the second carbon atom. So there is a double bond between both the carbon atoms.
Writing Lewis structure
The following procedures are used for writing the Lewis dot structures or Lewis structures:
Step 1: First, we determine the needed amount of electrons for drawing the structure by combining the valence electrons of the combining atoms. For Example, in methane, CH4 molecule, there are 8 valence electrons (of which 4 belongs to carbon while other 4 to H atoms) (in which 4 belongs to carbon while other 4 to H atoms).
Step 2: Each negative charge i.e. for anions, we add an electron to the valence electrons and for each positive charge that is, for cations we deduct one electron from the valence electrons.
Step 3: Using the chemical symbols of the combining atoms and creating skeletal structure of the compound, divide the total amount of electrons as bonding share pairs between the atoms in proportion to the total bonds.
Step 4: The center location in the molecule is taken by the least electronegative atom occupying Hydrogen and fluorine often occupy the terminal positions.
Step 5: After dispersing the shared pairs of electrons for single bonds, the leftover electron pairs are employed either for multiple bonds or they comprise lone pairs.
The primary criterion is that each bound atom obtains an octet of electrons.
Conclusion
Kossel and Lewis devised an electronic theory of valence or theory of chemical bonding to explain the creation of a chemical link between the two atoms. According to the electrical theory of valence, every atom seeks to acquire octet configuration (presence of eight electrons) in its valence shell by losing or acquiring or through sharing of electrons. This is known as the “Octet Rule”. The electrostatic forces of attraction that bind the two oppositely charged ions together are known as “electrovalent bonds”. Atoms gain stability via the aid of bond formation. The process of bond creation is connected with the decreasing energy of the system. Only valence electrons participate in chemical bonding, but not interior electrons. To comprehend the notion of valence electrons Lewis created the notion depicting valence electrons with dots, which are termed Lewis symbols.
Modern Periodic Table Periods
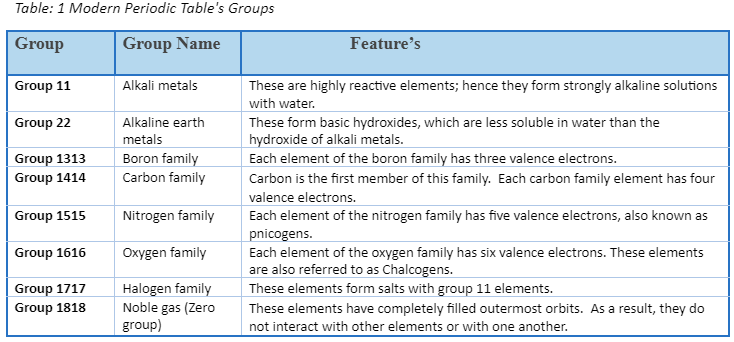
- In the modern or long form of the periodic table, periods are the horizontal rows.
- There are seven periods in the periodic table.
- From top to bottom, they are numbered 1, 2, 3, 4, 5, 6, and 7.
- Only two elements make up the first period: hydrogen and helium.
- The second and third periods each have eight elements.
- There are 18 elements in each of the 4th and 5th periods.
- The 6th period, on the other hand, has 32 elements.
- Four new elements have been added to the periodic table’s seventh period. 113-Nihonium, 115-Moscovium, 117-Tennessine, and 118-Oganesson are the elements. With 32 elements, this addition brings the 7th phase to a close.
- A separate panel at the bottom of the lengthy version of the periodic table. The lanthanoids are a group of 14 elements from the 6th era.
- The number of shells or energy levels in an atom of an element is represented by each period.
Types of elements in Modern Periodic Table
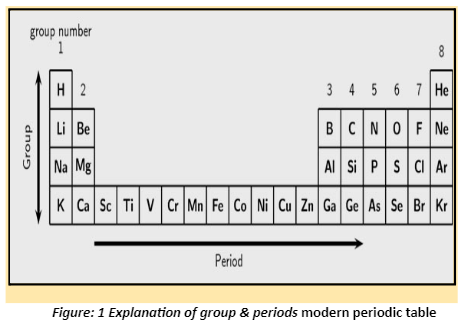
- Main group or representative elements: The elements found in groups 1 and 2 on the left side of the periodic table and groups 13 to 17 on the right side are referred to as main group or representative elements.
- Noble gases: These elements are found in Periodic Table Group 18 and have completely filled outermost shells, making them non-reactive.
- Transition elements: These elements are found in the middle block of the periodic table (Groups 3 to 12) and have two outermost shells that are incomplete; as a result, they change from most electropositive elements to most electronegative elements. As a result, they are referred to as transition elements.
- Inner transition elements: These are placed in two separate rows at the bottom of the periodic table to prevent the periodic table from expanding. There are two series, each with 14 elements. The first series, known as lanthanoids, contains elements 58 to 71. (Ce to Lu). The actinides are the second series of 14 rare-earth elements. It is made up of 90 to 103 elements (Th to Lr).
- Metals: Metals are located on the periodic table’s left side. Alkali metals are group one metals, and alkaline earth metals are group two metals.
- Non-metals: Non-metals are found on the periodic table’s right side.
- Metalloids: Metalloids are elements that exhibit both metal and nonmetal properties. They are found along the diagonal line beginning with group 13 (Boron) and ending with group 16. (Polonium).
Conclusion
Dmitri Mendeleev, a Russian chemist, created the framework for the modern periodic table in 1869, leaving gaps for elements yet to be discovered. If, after arranging the elements according to their atomic weight, he discovered that they did not fit into the group, he would rearrange them.
The periodic table is significant because it is organised to provide a wealth of information about elements and their relationships to one another in a single, easy-to-access reference. The table can be used to predict the properties of elements.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






