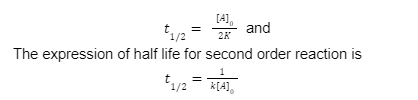Chemical kinetics is a field of physical chemistry that studies the rate of chemical processes. Such investigations also allow us to comprehend the process through which the response happens. As a result, in chemical kinetics, we can also calculate the rate including a chemical reaction.
The reactions are categorised into two classes based on their kinetic properties:
a) homogeneous reactions, which occur totally in one phase; b) heterogeneous reactions, which occur in more than one phase whether the transformation occurs on a catalyst’s surface or the walls of a container.
Definition of half life of a reaction
The time it takes for a reactant’s concentration to be lowered to one-half of its starting concentration is referred to as the half-life of a reaction.
It is worth noting that the half-life is independent of the starting concentration. Pick any moment in a reaction and one half-life from that point is where the reactant concentration is 1/2. In a first-order reaction, the reactant concentration declines by 1/2 in each sequence of evenly spaced time periods, i.e. t1/2.
We can obtain equations for the half lives for reactions of various orders by substituting the values t=t1/2 and [A]=1/2[A]0into the integrated laws
Half life of zero order reaction

Half life of first order reaction
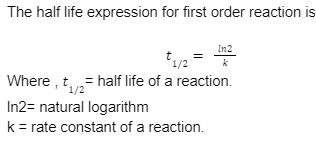
Half life of second order reaction
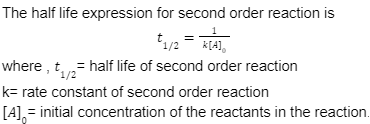
Calculation of half life of reaction

Zero order kinetics
The rate of a reaction in zero-order kinetics is independent of substrate concentration. To put it another way, increasing the amount of substrate does not increase the pace of the reaction. A zero-order reaction’s half-life is affected by both the starting concentration and the rate constant.
First order kinetics
The half-life depends solely on the reaction rate constant k.
Kinetics of second order
The half-life of second-order reactions is affected by both the initial concentration and the rate constant.
Conclusion
The half-life of a radioactive substance is the time it takes for one-half of its atoms to decay. The half-life of carbon-14 can be used by scientists to estimate the age of organic things. They calculate how much of the carbon-14 has been converted. They can then use this information to calculate the age of a drug. It is difficult to anticipate the decay of a single radioactive atom. The half-life of an atom does not explain the precise length of time that each and every atom spends before disintegrating. Understanding the notion of a half-life will alter your reading habits and how you spend your time. It will explain why our occupations are becoming more specialised and provide insight into how we may compete more successfully in a highly competitive world. Knowing about half-lives is useful because it allows you to identify when a radioactive material sample is safe to handle. The rule is that a sample is safe when its radioactivity falls below detection levels. That happens after 10 half-lives.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out