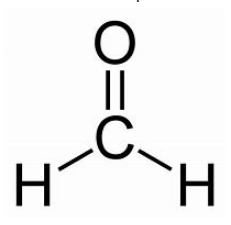
Formaldehyde is a colourless, strong-smelling gas used in making building materials and many household products. Formaldehyde Formula is represented as CH2O.
Formaldehyde-based glues are excellent bonding agents, giving high-quality performance while remaining cost-effective.Formaldehyde-based resins are used in a wide range of panel and board products in the wood products industry, allowing for the sustainable use of forestry resources and the reduction of waste. Composite wood panels, for example, are often created from recovered wood waste that would otherwise be burned or thrown away in a landfill.
Formaldehyde formula:
Methanal is the IUPAC name for Formaldehyde, and its condensed chemical formula is CH2O. An aldehyde is a functional group that consists of one carbon atom, two hydrogen atoms, and one oxygen atom. Formaldehyde has the chemical formula HCHO and is expressed as follows.
Formaldehyde, HCHO, has a molar mass of:
=(The atomic mass of carbon)+2x(The atomic mass of hydrogen)+ Atomic mass of oxygen
=(12.01)+2x(1.007)+15.999=30.023 g/mol
Hence, one mole of Formaldehyde weighs 30.023 grams.
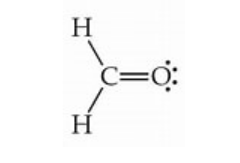
Lewis structure of formaldehyde:
The first member of the aldehyde functional group is methanal, which is made up of a single carbon atom that forms a double bond with an oxygen atom. Formaldehyde has a total of 12 valence electrons in its Lewis structure. The distribution of these electrons is as follows:
Skeletal structure of formaldehyde:
Formaldehyde is made up of a carbon atom that is joined to an oxygen atom by a double bond. The remaining electron-sharing spaces in carbon are occupied by two hydrogen atoms. There are three sigma bonds and one bond in it. Formaldehyde’s skeleton structure is depicted below:
Physical properties :
Particulars | Properties |
Appearance | Colorless gas |
Odour | Pungent |
Density | 0.8153 g/cm3 |
Melting Point | −92 degree celsius |
Boiling Point | −19 degree celsius |
Solubility in Water | 400 g/L |
Molecular shape | Trigonal planar |
Chemical properties:
- Because of the C=O bond in the CHO functional group, formaldehyde is polarised.
- The presence of positively and negatively charged centres aids nucleophilic molecules in attacking the electron-poor carbonyl carbon. Electrophiles attack the negatively charged oxygen atom at the same time.
- In the presence of ambient oxygen, formaldehyde is easily converted to formic acid.
- Electrophilic aromatic substitution reactions with aromatic chemicals produce hydroxymethylated derivatives from formaldehyde.
- In the presence of basic catalysts, formaldehyde participates in the Cannizzaro reaction, which produces formic acid and methanol.
Uses of formaldehyde:
The following are some of formaldehyde’s applications:
- It’s used in the production of resins such as urea-formaldehyde and plastics like Bakelite.
- It’s used to make dyes like indigo and pararosaniline, among other things.
- Formalin is a 40 percent aqueous solution of formic acid. It’s used as an antiseptic, disinfectant, germicide, and fungicide, as well as to keep animal specimens clean and sterilise surgical instruments.
- It’s utilised in barrel dyeing as a decolorizing agent.
- It’s used to make silvered mirrors.
- It’s used in the anti-polio vaccine’s manufacturing process.
Phenol formaldehyde resin:
Any of a number of synthetic resins manufactured by reacting phenol (an aromatic alcohol generated from benzene) with formaldehyde, is commonly known as phenolic resin (a reactive gas derived from methane) or phenol formaldehyde resin. The first entirely synthesised polymers to be commercialised were phenol-formaldehyde resins. Bakelite, a trademarked phenolic resin, transformed the market for moulded and laminated parts for use in electrical equipment in the first decades of the twentieth century. Phenolics are still important industrial polymers, with adhesives for the bonding of plywood and other structural wood products being the most typical application.
There are two major processes for converting polymer into usable resins in industrial usage. In one technique, an excess of formaldehyde is reacted with phenol in aqueous solution in the presence of a base catalyst to produce a resole, a low-molecular-weight prepolymer. The resole can be cured to a solid thermosetting network polymer by sandwiching it between layers of wood veneer and then heating the assembly under pressure to make plywood.
The other approach involves using an acid catalyst to react formaldehyde with an excess of phenol. This technique yields a solid prepolymer known as a novolac (or novolak), which is similar to the final polymer except for its lower molecular weight and thermoplastic properties (that is, it can be softened by reheating without undergoing chemical decomposition). Grinding the novolac to a powder, mixing it with fillers like wood flour, minerals, or glass fibres, then heating the combination in a pressure mould is how it’s done. Novolacs require the addition of additional formaldehyde or, more typically, chemicals that disintegrate into formaldehyde when heated in order to cure to a thermosetting resin.
Conclusion:
In medical labs, formaldehyde is commonly used to preserve specimens. Formaldehyde is an acidic substance. It’s also worth noting that formaldehyde is sometimes referred to as Methanal. It’s called Methanal since it’s made from formic acid. Its urea-phenol combination is a critical polymer widely utilised in adhesives and the motor sector. As a decolorizing chemical, formaldehyde is used in barrel dyeing. It’s also used in the silvering of mirrors as a key component. It does, however, have significant drawbacks. If formaldehyde is retained at greater concentrations, it can cause headaches, a burning sensation in the throat, and other problems.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out