The Finkelstein reaction is used to make alkyl halides and haloalkanes.The Finkelstein reaction is often referred to as the halogen exchange reaction or the halex reaction.The Finkelstein reaction has the following formula:
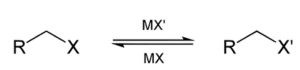
Alkyl iodides are formed when bromide, Nal, alkyl chloride, and dry acetone combine.
CH3CH2-Br + NaI → CH3CH2-I + NaBr
If red mercuric and iodine are utilized, alkyl iodide can be made from acids. Aliphatic carboxylic acids can be converted into esters and alkyl halides. The chemical equation for a reaction like this is:
RCOOH + R (COO)4Pb → X2 → RCOOR + 2RX
Finkelstein Reaction and Swarts Reaction are two types of reactions that occur as a result of halogen exchange. Swarts Reaction is the most efficient approach to make alkyl fluorides by heating bromide or alkyl chloride in the presence of metallic fluorides such as AgF, Hg2F2, CoF2, or SbF3 .
Condition for finkelstein reaction:
- Because of the following reasons, the Finkelstein reaction will not occur if metal bromide or metal chloride is used as a substitute for metal iodide and alkyl iodide is used instead of alkyl bromide or alkyl chloride.
- Acetone, as a polar aprotic solvent that can dissolve covalent molecules, can be regarded as a covalent compound.
- All metal halides, including NaCl, Nal, and NaBr, are ionic compounds. When a large anion and a small cation are involved, a higher covalent compound can be created.
- In NaCl and Nal, the cation Na+ remains unaltered, but the anion Cl-, Br-, and l- in NaCl, Nal, and NaBr vary in size.Because anion I- (iodide) is bigger, Nal has the highest covalent character.The other two metal halides, NaBr and NaCl, are insoluble in acetone, whereas Nal is easily dissolved.
As a result, the Finkelstien reaction is influenced by a number of factors, including:
- Halogen-carbon bond
- Nature of the group
- Reactivity of alkyl halides in groups
- Nucleophilicity
Advantages:
The finkelstein response has the following advantages:
- Alkyl iodide synthesis that is suitable.
- The amount of Nal in acetone can be used as a qualitative test to determine an alkyl halide’s class.
- The capability with which alkyl halides conduct the Finkelstein reaction varies greatly, which is why it is utilized for analysis.
Examples of finkelstein reaction:
- Sodium iodide and methyl bromide react together.
CH3Br + NaI → CH3I + NaBr
- The reaction of sodium iodide with ethyl bromide.
CH3CH2Br + NaI → CH3CH2I + NaBr
- The reaction between sodium iodide and ethyl chloride.
CH3CH2Cl + NaI → CH3CH2I + NaCl
Applications:
- It is utilized in the investigation of alkyl halides since the ease with which they conduct the reaction varies substantially.
- Used to make alkyl iodides quickly and easily.
- Chrysochlamic Acid is made with this ingredient.
- The reaction of NaI in acetone can be used to determine the halide class of an unknown halide.
Important points in finkelstein reaction:
- Finkelstein’s reaction depicts the substitution of one halogen for another.Because of the different solubility of metal halide salts in acetone, halide exchange is a reversible process.
- The rate of reaction of electron donors on the alkyl halide increases, whereas the rate of reactivity of electron-withdrawing groups drops.
- In the modified Finkelstein reaction, alcohol is first transformed into a mesylate or tosylate, then treated with metal halide to obtain the required alkyl halide.
- The reaction works flawlessly because mesylate and tosylate are adequate withdrawing groups.
- When combined with diamine ligands, cul copper (I) iodide is the only suitable catalytic method for the aromatic Finkelstein reaction.
Conclusion:
In terms of utility, this reaction can be used to convert an alkyl chloride or alkyl bromide into an alkyl iodide. The treatment of a sodium iodide solution in acetone is part of this process. This is due to the fact that sodium iodide dissolves in acetone whereas sodium chloride and sodium bromide do not. Due to mass action, the less soluble sodium chloride and sodium bromide tend to precipitate, resulting in sodium iodide as the final product. Furthermore, utilizing allyl and benzyl halides, we can observe an unusually high yield. The reaction with secondary halides, on the other hand, is quite slow.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



