Introduction –
Organic chemistry is an essential branch of chemistry that generally deals with the compounds and reactions that include carbon atom(s). The rules governing this branch make organic chemistry so interesting and sometimes challenging for students to wrap their minds around. Organic chemistry has over 15 million compounds and is increasing more and more with discoveries in this field. Each of these compounds is ruled by slightly or completely autonomous rules governing the type of reaction they might undergo. Not only that, the rules can change depending on the slight change in the compounds.
Now let us discuss some of the critical rules regulating organic chemistry working.
Carbon Tetravalence
Carbon stands out as one of its kind elements in the complete periodic table. Its flexibility to make the most unusual bonds with many other elements makes it unique. Tetravalency is one trait that makes it possible for carbon to make multiple bonds. Tetravalency means that carbon has a valency of 4. This allows carbon to make four bonds with itself and other elements such as F, Cl, Br (etc.) to form the most compounds.
Structural representation
An organic compound can very well be represented both as a 2-dimensional and a 3-dimensional structure. These have their subcategories as follows:
2D structure
- i) Complete structural representation – This is the type of representation of an organic structure that shows the atoms of carbon and the adjacent atoms bonded to each other through lines via single, double, or even triple bonds. This helps in understanding the two ways the atoms are attached are functional in a 2D plane.
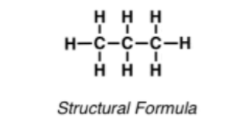
ii) Condensed structural representation – This is the structural representation of an organic compound in which the atoms are not shown through any line bond. However, the atoms of the same family are shown together, with a number assigned to show the number of those atoms at their foot. This representation is helpful in long/short-form text-heavy sentences. Though this kind of representation might not accurately represent the real-life compound, it is generally used by students in tests and examinations.
iii) Bond-line structural representation – This type of structural representation of a compound is a hybrid of the above two mentioned structural representations. Here, the atoms linked in a pocket of a branch are condensed and represented in the form of condensed structural formula. In contrast, the mainline/branch is represented in a usual way. This representation depicts in a more accurate and more composed form for trying to understand the physical nature of the compound.
The 3-dimensional representation, as the name suggests, is the actual, real-life depiction of the compound. The 3D depiction of a compound on a 2-dimensional paper can be done with the help of the “Solid and dashed wedge” method. In these methods, the line between 2 atoms can be represented as a solid wedge if the atom is near the observer. In contrast, the dashed wedge represents the bond in which the atom is away from the observer.
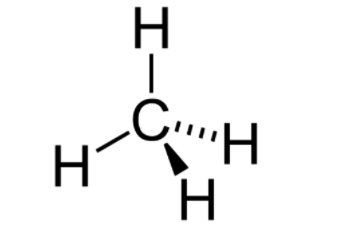
Isomerism
Isomerism is when multiple organic compounds have the same chemical formula, but they widely differ in structural representation. This means, for example, if C6H15 is the chemical formula for a compound, the structural representations can result in 2, 3, or even more compounds.
There are sub-categories to isomerism as discussed below –
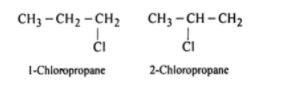
i) Position isomerism – This is the type of isomerism in which the chemical formula for the compound is the same. However, a compound’s nature and chemical properties are determined by the subsequent positioning of an atom/functional group attached to the main carbon chain.
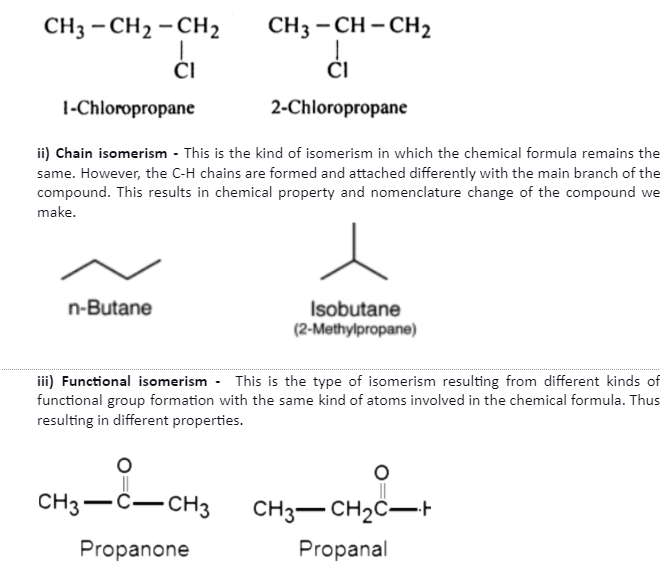
Conclusion
The world of organic chemistry is vast and continues to be ever-expanding. Having discussed some of the crucial and basic principles related to organic chemistry, one must get a rough idea about the subject and its vitality in our day-to-day life. The principles ruling the chemical reactions related to organic chemistry vary from compound to compound. Even then, some of these basic principles come in handy to better understand or sometimes articulate one’s reading and research. However, it is crucial to keep yourself up to date with the way this field is incessantly expanding and growing in the scientific community.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out