Introduction
The phenomenon of boiling point elevation happens when the boiling point of a liquid (a solvent) is enhanced by the addition of another substance, resulting in a solution with a higher boiling point than the pure solvent. When a non-volatile solute is introduced to a pure solvent, the boiling point rises.
While the amount of dissolved particles in a solution affects boiling point elevation, their identification is not a factor. Interactions between solvents and solutions have little effect on boiling point elevation.
An ebullioscope is used to correctly measure boiling point and so determine whether boiling point elevation has happened or not, as well as how much the boiling point has changed.
What is Boiling Point Elevation?
The boiling point elevation is a colligative feature, which means that it is affected by the presence and amount of dissolved particles but not by their identity. It is a result of solvent dilution in the presence of a solute. It is a phenomenon that occurs for all solutes in all solutions, even for perfect solutions, and is unrelated to any unique solute-solvent interactions. Whenever we take the solute that is an electrolyte, like different salts, or we take a nonelectrolyte, the boiling point rises. The origin of the boiling point elevation is entropic in thermodynamic terms and may be described in terms of the solvent’s vapour pressure or chemical potential.
The explanation in both circumstances is based on the fact that many solutes are only present in the liquid phase and do not enter the gas phase (except at extremely high temperatures).
How is Boiling Point Elevation used in real life?
Boiling point of water that contains salt is always greater than that of the pure water. Because salt is an electrolyte that dissociates into ions in solution, it has a significant effect on boiling point. It is important to note that nonelectrolytes, such as sugar, raise the boiling point. A nonelectrolyte, on the other hand, has less of an effect per mass than a soluble electrolyte since it does not break into many particles.
Equation of Boiling Point Elevation
The formula for calculating boiling point elevation is a mixture of the Clausius-Clapeyron equation and Raoult’s law. It is assumed that the solute is non-volatile.

Where,
Tb is the boiling point elevation.
Kb is the ebullioscopic constant that varies according to the solvent.
m is molality of the solution
As a result, the boiling point elevation of a chemical solution is precisely proportional to its molal concentration.
Elevation of Boiling Point and Vapour Pressure
A liquid’s boiling point is defined as the temperature at which the vapour pressure equals air pressure. Because the vapour pressure of a solution is smaller than that of the solvent, at higher temperatures the vapour pressure of the solution will equalise with the atmospheric pressure. In other words, the solution’s boiling point will be greater than the solvent’s. The pure solvent and solution vapour pressure-temperature graphs clearly demonstrate the solution’s higher boiling point as compared to the pure solvent.
So, boiling point elevation is the phenomenon that happens when the boiling point of a liquid is enhanced by the addition of another fluid, resulting in a solution with a higher boiling point than the pure solvent. When a non-volatile solute is introduced to a pure solvent, the boiling point rises.
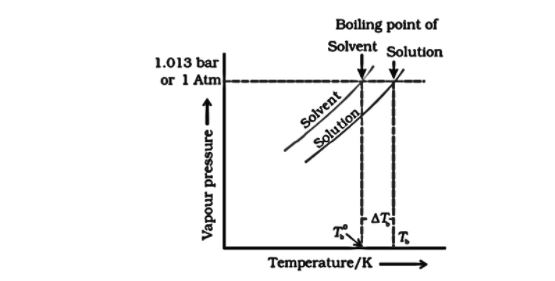
The dotted horizontal line corresponds to a pressure of 1.0 atm. It crosses the vapour pressure curves at two places, T0 and T. The boiling point of the solution, T, is higher (elevated) than the boiling point of the solvent, T0. Thus, the increase in boiling point of a solution is a direct result of the decrease in vapour pressure caused by solute addition.
What Is the Process of Boiling Point Elevation?
In a simple word, boiling point rises because the majority of solute particles remain in the liquid phase rather than entering the gas phase. To boil, the vapour pressure of a liquid must surpass the ambient pressure, which becomes more difficult to achieve when a nonvolatile component is added. You can think of adding a solute as diluting the solvent if you like. It makes no difference whether the solute is an electrolyte or not. For example, whether you add salt (an electrolyte) or sugar, the boiling point of water rises (not an electrolyte).
What Causes Boiling Point Elevation?
Because the addition of a non-volatile solute reduces a solution’s vapour pressure, the boiling point of a solution is higher than that of the solvent, requiring it to be heated to a higher temperature to achieve a vapour pressure equal to atmospheric pressure. As a result, the solution boils faster than the pure solvent.
Conclusion
The phenomenon of boiling point elevation happens when the boiling point of a liquid is enhanced by the addition of another substance, resulting in a solution with a higher boiling point than the pure solvent. When a non-volatile solute is introduced to a pure solvent, the boiling point rises. While the amount of dissolved particles in a solution affects boiling point elevation, their identification is not a factor. An ebullioscope is used to correctly measure boiling point and so determine whether boiling point elevation has happened or not, as well as how much the boiling point has changed. The boiling point elevation is a colligative feature, which means that it is affected by the presence and amount of dissolved particles but not by their identity. When a non-volatile solute is introduced to a pure solvent, the boiling point rises.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out



