Introduction to Dalton’s law of partial pressures
As per Dalton’s law, we understand the relationship between two gases in a container.
Dalton’s law of partial pressures has a lot of applications.It is one of the most conventional methods to measure the pressure exerted by two gases and calculate the partial and total pressures.
With the help of this law, we understand that pressure in a container consisting of a mixture of gases could be found by calculating the sum of partial pressures of each of these gases.
The partial pressure between these gases is exerted individually only when they occupy the volume of the mixture at the same temperature.
So we can say Dalton’s law allows us to find the pressure exerted by two gases in a container.
While you are asking your local tire shop owner to check the pressure of your tires, have you ever thought about what he does? You might have noticed that they use a tire gauge to measure the pressure in the tube of your tire.
Why exactly?
Because it helps them measure and adjust the physical properties of a large number of gases in our tires and maintain the right pressure, that’s not possible to tell by some other method.
On this kind of molecular level, the pressure that we are measuring comes from the force of the individual gas molecules that collide with other objects (in this case, the walls of the tube of the tire.) This is where Dalton’s law of partial pressures is implemented.
John Dalton, an English chemist, stated “Dalton’s law of partial pressure”.
Dalton’s law (or Dalton’s law of partial pressure) has been adopted as the universal method to calculate the partial and the total pressure of gases.
Dalton’s law also doesn’t assume any chemical interaction between the component gases.
So let’s understand the explanation of Dalton’s law of partial pressure in detail.
The Partial Pressure of a gas
The entire thought about this theory occurred to John Dalton when he was on vacation, and the weather was too humid. This made him question the humidity and the particles in the air.
In this observation, he realised that each gas has a distinct behaviour in a mixture of gases. The observation could be summarised in the following statement –
If there’s a container that contains 10 Nitrogen molecules, they will exist independently. If we were to add 10 oxygen molecules to this container, the pressure would increase to 20 mmHg. Now, if we were to remove 5 molecules of oxygen from this container, we would have 10 Nitrogen molecules and 5 oxygen molecules, and the pressure would decrease to 15 mmHg.
Even though the number of particles is changed, every particle will still fly around and hit the walls of the container, contributing individually to the pressure of the container yet not interfering with other particles at all. This means the more gas particles there are in a container, the higher the pressure is in that container. At the same time, the lower particles, the lower pressure.
The individual pressure from both these gases refers to partial pressure.
What is Dalton’s law of partial pressures – An explanation
As Dalton understood partial pressures, the phenomenon was named after him.
According to the law of Dalton, we can conclude that a mixture filled with multiple gases exert partial pressures on each other. The total pressure equals the sum of partial pressures that each gas exerts.
This fundamental could also be illustrated with a figure.
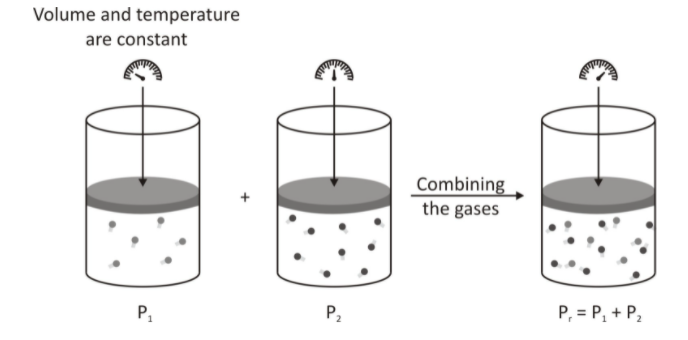
This partial pressure that’s given out by both these gases present in container 1 and 2 is the pressure that could be exerted by these gases individually if they occupied the volume of the mixture at the same temperature.
In such a case, a single gas, without affecting the pressure, expands to fill the container. By this behaviour among the gases, we find out that a single gas’s pressure is dependent on the number of moles this gas has.
Not only that, but it is equally dependent on the volume and the temperature of the system.
However, the different gases have the same volume and temperature. Due to the reason that each gas in the figure adds its pressure on the system, it could be summarised in an equation that allows us to find the total pressure of this mixture of gases. This can be illustrated by:
Ptotal = P1 + P2 + …. + Pn
In this equation,
Ptotal is the total pressure exerted by the mixture of the gases, P1, P2,…, Pn is the partial pressures of the first, and second gases and n is the mixture of ‘n’ gases in the container.
Partial Pressures in Terms of Mole Fraction
When we have a mixture of gases, the mole fraction of one gas equals the ratio of partial pressures of the gas to the total pressure that gets released by the gaseous mixture.
Mole fraction also has another implementation. It also helps us find out when the total number of moles in the mixture has already been specified to us the total number of moles of a constituent gas.
To illustrate the same in an equation, Pi
Xi = Pi / Ptotal = Vi / Vtotal = ni/ ntotal
Here,
Xi is the mole fraction of a gas
i is the mixture of ‘n’ gases
n denotes the number of moles
P is the pressure
V is the volume
Conclusion
Dalton’s law of partial pressure has a variety of applications. It’s even used in processes like steam distillation, calculating the pressure of dry gas, or even calculating the equilibrium constant in terms of partial pressure (Kp).
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






