Carbohydrates, also known as saccharides, are the most numerous class of biomolecules on the planet. As the name suggests, Carbohydrates are biomolecules formed with carbon and water. It usually degrades the animal’s body, releasing energy.
Carbohydrates
As the name suggests, Carbohydrates are biomolecules formed with carbon, hydrogen and oxygen. A carbohydrate is a naturally occurring compound or a derivative of one with the general chemical formula Cx(H2O)y. Carbohydrates are the most common chemical components required for all living things to exist. Sources of Carbohydrates include fresh fruits, vegetables, corn, potatoes, milk, and milk products. Soda, white bread, artificial sugar, pastries, and other highly processed meals are unhealthy sources. Sources of carbohydrates can be plant-based as well as animal-based.
Carbohydrates are classified as simple or complex types:
Simple Carbohydrates
Simple carbohydrates are generally there in cold drinks, biscuits, chocolates, etc. they include processed sugar. Simple carbohydrates also include naturally occurring sugars, like fruits. These are called simple because they break easily, causing problems in the body.
Complex Carbohydrates
Complex carbohydrates are an important source of energy, and they are important for body function. They are polysaccharides and take time to break down; they provide energy for a long time hence being important.
Classification of Carbohydrates:
Although there are several classification schemes for carbohydrates, the separation into four primary groups—monosaccharides, disaccharides, oligosaccharides, and polysaccharides—is one of the most widely used. The three most common simple sugars—glucose, fructose, and galactose—all have the same molecular formula (C6H12O6), but their atoms are arranged differently, giving them various properties; thus, they are isomers.
(i) Monosaccharides:
A carbohydrate that can not further form polyhydroxy aldehyde or ketone when reacted with water is a monosaccharide. There are around 20 monosaccharides found and studied. Some common illustrations are glucose, fructose, ribose, etc.
(ii) Oligosaccharides:
Carbohydrates that yield 2 to 10 monosaccharide molecules are called oligosaccharides. Then they can be di-, tri-, or tetra-saccharide, depending upon the number of monosaccharides they have. The most common are disaccharides which are made up of 2 monosaccharide units. They may be identical or distinctive. For illustration, one molecule of sucrose on hydrolysis gives one molecule of glucose and one molecule of fructose, whereas maltose gives two molecules of exclusive glucose.
(iii) Polysaccharides:
Carbohydrates that give many monosaccharide units, like 20-30 in number, are generally known as polysaccharides. Some familiar illustrations which can be seen in daily life are starch, cellulose, glycogen, epoxies, etc. Polysaccharides in general, aren’t that sweet in flavour, so they’re also known as non-sugars.
Classification based on reducing and nonreducing sugar
The carbohydrates may also be categorised as either reducing or nonreducing sugars.
Reducing sugar is those carbohydrates that Tollen, Fehling, and Benedict test. All monosaccharides are reducing sugar.
All those carbohydrates that do not give Tollens, Fehling or benedict’s test are nonreducing sugar. Example: Starch, cellulose, glycogen, sucrose, etc.
Monosaccharides
Monosaccharides are carbohydrates that cannot be broken into two simpler carbohydrate molecules. Monosaccharides are more classified on the root of the number of carbon molecules and the functional group present in them.
The most nutritionally consequential and plenteous monosaccharide is glucose, which is harnessed as the significant cell energy in the mortal body and can be found unrestrained in body muscles and fluids.
Some monosaccharides
Glucose
Glucose occurs voluntarily in nature as well as in the associated form. Glucose is aldohexose and is also comprehended as dextrose. It’s the monomer of numerous of the larger carbohydrates, such as cellulose. It’s presumably the most plenteous organic compound on the globe. Its molecular formula was found to be C6H12O6.
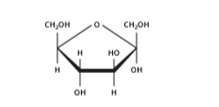
Fructose
Fructose is a significant ketohexose. It’s obtained along with glucose by the hydrolysis of the disaccharide sucrose. Fructose-like glucose has a molecular formula C6H12O6. At the root of its chemical reactions, we concluded that it bears a ketone functional group at carbon positions two and six carbons in the straight-chain same as glucose.
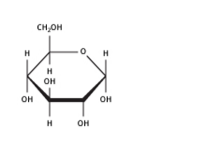
Disaccharides
A disaccharide is the sugar formed when a glycosidic reaction joins two monosaccharides. They are adjoined together by an oxide bond formed by removing a water molecule from two monosaccharides. A bond between two monosaccharide molecules through an oxygen atom is called glycosidic relation. In disaccharides, if the reducing groups of monosaccharides, i.eAldehyde or ketone groups, are there, these are nonreducing sugars, e.g., sucrose.
Some Disaccharides
Sucrose
One of the familiar disaccharides is sucrose which on hydrolysis gives an equimolar mixture of D-glucose and D- (-)fructose. These two monosaccharides are gripped together by a glycosidic linkage between CARBON 1 of α-D-glucose and C2 of β-D-fructose. Since the reducing groups of glucose and fructose are present in the glycosidic bond arrangement, sucrose is a nonreducing sugar.
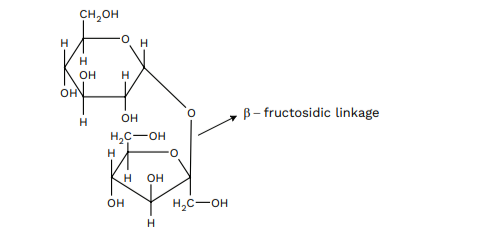
Maltose
Maltose is composed of two α-D-glucose units in which CARBON 1 of one glucose (I) is linked to CARBON 4 of another glucose unit (II). The independent aldehyde group can be formed at CARBON 1 of alternate glucose as a result, and it shows reducing traits, so it’s a reducing sugar.
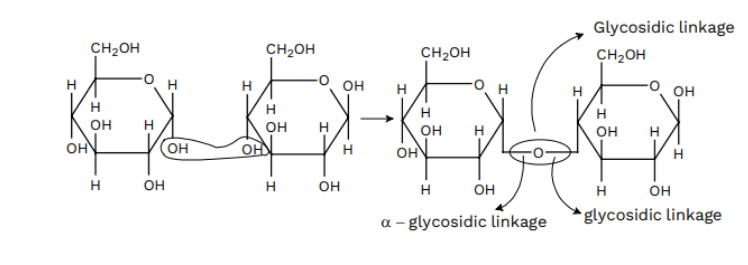
Lactose
It’s more generally comprehended as milk sugar since this disaccharide is in milk. It’s crafted of β-D-galactose and β-D-glucose. The linkage is between CARBON 1 of galactose and CARBON 4 of glucose. An independent aldehyde array may be present at CARBON 1 of the glucose unit, so it’s also a reducing sugar.
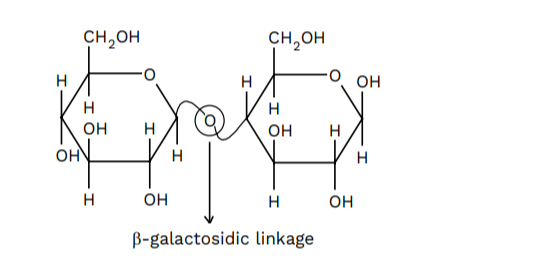
Polysaccharides
Polysaccharides bear a big number of monosaccharide units joined together by glycosidic linkages. These are the most generally found carbohydrates in nature. They are substantially portrayed as food storehouses or structural stuff.
Example: Starch
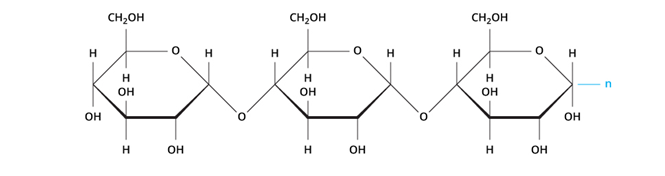
Plants use starch as the primary carbohydrate to store glucose for subsequent usage as energy. Sources of starch include rice, beans, wheat, maise, potatoes, etc.
When humans consume starch, an enzyme called amylase, which is found in saliva and the intestines, breaks the connections between the repeated glucose units, allowing the sugar to enter the bloodstream. The human body either transports glucose to the locations where it is needed for energy or saves it as its specific polymer, glycogen, once released into the bloodstream.
Conclusion
Carbohydrates are originally birthed by plants and form a veritably big group of inherently occurring organic compounds. They have been broadly divided into three groups: monosaccharides, oligosaccharides, and polysaccharides. We can classify them as reducing and nonreducing sugar as well.
Monosaccharides are more classified on the root of the number of carbon molecules and the functional group present in them. A disaccharide is the sugar formed when two monosaccharides are joined by glycosidic relation.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






