A typical atom is made up of three subatomic particles: protons, neutrons, and electrons, which are arranged in a symmetrical arrangement (as seen in the helium atom below). There are other types of particles, such as alpha and beta particles, that exist (which are discussed below). The Bohr model is a straightforward representation of the three fundamental subatomic particles. The nucleus of an atom contains the majority of its mass, which is a small, dense area at the centre of every atom that is made up of nucleons. Protons and neutrons are examples of nucleons. The nucleus of an atom contains all of the positive charges that exist in the atom, and this charge is derived from the protons. Neutrons are charged in a neutral manner. It is outside of the nucleus that electrons can be found, as they are negatively charged.
The Bohr model is out of date, yet it is nevertheless useful because it displays the three fundamental subatomic particles in a straightforward manner. Electron clouds are more precise depictions of the locations where electrons are found than other types of representations. Where the electrons are more likely to be found is represented by darker areas, and where the electrons are less likely to be found is represented by lighter areas.
- The atomic mass unit is represented by the symbol Au in the International System of Units.
- The proton’s positive charge cancels out the electron’s negative charge, resulting in a neutral state. Neutrons are devoid of charge.
- Protons and neutrons are quite similar in terms of mass, and they have a significantly greater mass than electrons, which are much lighter. When compared to the masses of neutrons and protons, the mass of an electron is typically insignificant.
- A particle’s spin is connected with its rotational motion. Protons, neutrons, and electrons each have a total spin of 12 compared to the rest of the nucleus.
Subatomic Particles
Long ago, it was believed that atoms were the most fundamental particles that matter is made up of, and that these atoms could not be further divided into smaller units. The experiments carried out in the later half of the nineteenth century and the early years of the twentieth century demonstrated that the atom is not the ultimate particle, as was previously believed. The scientists’ never-ending efforts resulted in the discovery of subatomic particles throughout the universe.
The three fundamental subatomic particles that make up an atom are depicted in the diagram below.
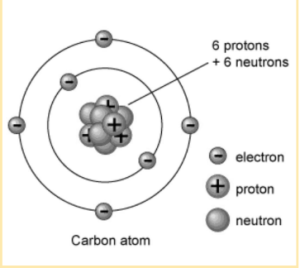
In order to explain certain facts, Dalton’s atomic theory had several restrictions, which served as a springboard for the discovery of electrons and protons. The existence of neutrons was discovered through further inquiry. Subatomic particles, which are the building blocks of atoms, are generally composed of protons, electrons, and neutrons, among other elements.
Protons
When Ernest Rutherford conducted his gold foil experiment in the year 1919, he became the first person to discover protons. Alpha particles (helium nuclei) were projected onto gold foil, and the positive alpha particles were deflected as a result of this. He came to the conclusion that protons exist in a nucleus and that they have a nuclear charge that is positive. Protons and neutrons combine to form the nucleus of an atom, which is why they are referred to as nucleons. The following are some significant aspects to remember about the discovery and properties of protons, as well as their history.
- Protons are subatomic particles that have a positive charge.
- When it comes to atoms, the number of protons equals the number of electrons in the atom.
- Ernest Rutherford is credited with making the discovery of protons.
- The removal of one electron from a hydrogen atom can result in the production of protons.
- A proton has a mass of 1.676 * 10-24 g.
- A proton has a charge of +1.602 * 10-19 Coulombs.
Electrons
Sir John Joseph Thomson made the groundbreaking discovery of electrons in 1897. J.J. Thomson demonstrated the relationship between the mass of cathode rays and their electric charge after conducting numerous experiments with cathode rays. In his experiments, he verified that cathode rays are fundamental particles with a negative charge; hence, electrons were given the name cathode rays. Robert Millikan discovered the value of the electric charge through the use of oil drop experiments.
Electronically charged particles are found in an electron cloud, which is the region around the nucleus of an atom. The probability of discovering an electron near the nucleus of an atom is usually higher the closer the atom is to the nucleus. Electrons are denoted by the symbol e-. In comparison to protons, electrons have a negative charge that is equal in magnitude to the positive charge of electrons. Their mass, on the other hand, is far less than that of a proton or neutron (and as such is usually considered insignificant). Ions are formed when protons and electrons are in unequal amounts, resulting in either positive cations or negative anions.
Neutrons
When James Chadwick demonstrated in 1932 that penetrating radiation contained beams of neutral particles, he became the first person to find neutrons in the universe. Neutrons are found in the nucleus with the protons, where they form the nucleus. As a result of their interaction with protons, they account for nearly all of the mass of the atom. The neutron number is the number of neutrons present in an atom, and it may be calculated by subtracting the proton number from the atomic mass number. The number of neutrons in an element determines the isotope of an atom, as well as its stability in many cases. The number of neutrons does not have to be the same as the number of protons in order to be equal.
Conclusion
Therefore, it concluded that Subatomic particles play two vital roles in the structure of matter. They serve as the fundamental building pieces of the universe as well as the mortar that holds the bricks together. Despite the fact that the particles that perform these two distinct functions are of two distinct sorts, they do share several properties in common, the most notable of which is their size. It’s probable that subatomic particles are made up of a mass-core surrounded by a charge-mantle, which would explain their mass. The mass-core is composed of mass-substance,’ has a fixed density, and has a variable volume.
 Profile
Profile Settings
Settings Refer your friends
Refer your friends Sign out
Sign out






